Shuo Han group develops antigen amplification technology for cancer immunotherapy
Source:Shuo Han
2025-11-20
On September 10, the international academic journal Nature published online the latest research from Professor Shuo Han’s team at the CAS Center for Excellence in Molecular Cell Science (CAS Institute of Biochemistry and Cell Biology). The paper, titled “Amplifying antigen-induced cellular responses with proximity labelling,” reports a brand-new strategy for cancer immunotherapy. Its core concept is to use proximity-labeling technology to construct artificial antigen clusters in situ and at high density on the surface of target cells. The researchers employed a red-light- or ultrasound-activatable engineered nanoenzyme (PCN), which catalyzes a covalent reaction between a probe containing an artificial antigen (FITC) and neighboring proteins on tumor-cell surfaces, thereby “amplifying” the target antigen signal (PATCH). This innovation addresses two critical bottlenecks in immunotherapy: insufficient antigen density and poor targeting specificity.
Cancer immunotherapy—especially approaches that harness T cells to recognize and eliminate malignant cells—has become a major breakthrough in oncology. However, its broad application faces two major obstacles. First, many tumor-associated antigens are expressed at low density on cancer-cell surfaces, making it difficult to effectively activate T cells. Second, these antigens are often expressed at low levels in normal tissues as well, leading to poor specificity and potential off-target toxicity. Thus, strategies capable of strengthening antigen signals while precisely targeting tumor cells are urgently needed.
To tackle this challenge, the research team approached it from a chemical-biology perspective, asking whether proximity-labeling—originally developed as a technique to detect spatially adjacent proteins—could be transformed into a functional regulatory tool, capable of directly amplifying targeting signals on tumor-cell surfaces and thereby “labeling” the cells that should be eliminated by the immune system.
In this study, the authors applied proximity labeling to immune regulation for the first time and developed a completely new strategy for engineering cell-surface proteins, termed Proximity Amplification and Tagging of Cytotoxic Haptens (PATCH). At the center of this strategy is an engineered nanoenzyme (PCN) that can be selectively activated by red light or ultrasound. The nanoenzyme is first delivered to tumor-cell surfaces, and then precisely and non-invasively activated via external red light or ultrasound. Once activated, it catalyzes probes containing an artificial antigen (FITC) to rapidly and massively form covalent bonds with cell-surface proteins within a few nanometers of the nanoenzyme. This process effectively “plants” high-density artificial antigen clusters on the surface of target tumor cells.
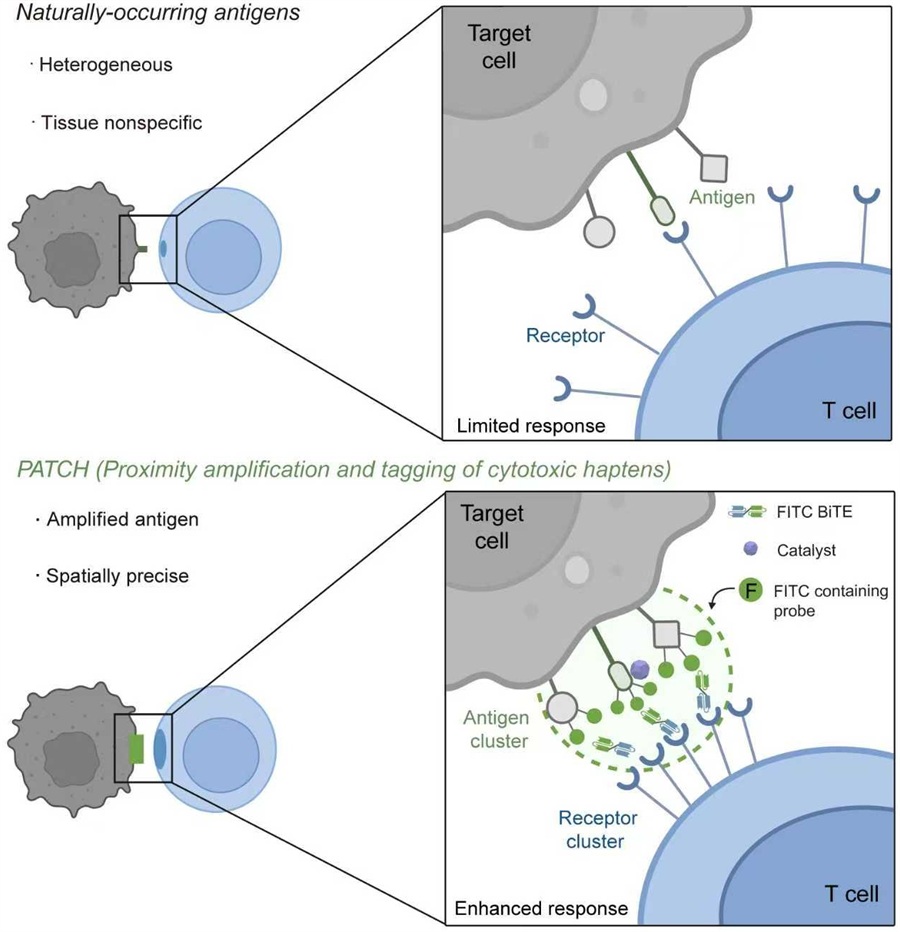
These in situ constructed antigen clusters act as “super beacons” for immune cells. Using a bispecific T-cell engager (BiTE) that binds both FITC and CD3 on T cells, the antigen clusters efficiently recruit and cluster T-cell receptors (TCRs), strongly activating T cells and drastically boosting their ability to recognize and kill tumor cells.
The PATCH strategy produced striking therapeutic effects across multiple solid-tumor animal models and patient-derived tumor samples. The study showed that PATCH not only eradicated treated tumors completely, but—importantly—the robust tumor-killing process released abundant tumor antigens, which in turn triggered systemic immune responses against distant, untreated tumors (the “abscopal effect”). It also generated durable immune memory, effectively preventing tumor recurrence.
This work is the first to expand proximity-labeling chemistry from a tool for detecting molecular interactions into a powerful functional regulatory technology. Its key strengths include solving the problem of insufficient natural antigen density through catalytic amplification, and ensuring high therapeutic specificity through precise physical control (light or ultrasound activation). This significantly broadens the landscape of targetable tumor antigens and provides a new conceptual and technological foundation for next-generation immunotherapies that are precise, potent, and low-toxicity.
Professor Shuo Han (CAS Center for Excellence in Molecular Cell Science) and Professor Qiang Gao (Zhongshan Hospital, Fudan University) are co-corresponding authors of the paper. The co-first authors are postdoctoral fellow Shuojun Li (CECCM), PhD student Yinghui Men (Shanghai Jiao Tong University), PhD student Zihan Wang (CECCM), and PhD student Yingcheng Wu (Fudan University). The study was supported by researchers Chenqi Xu and Guangchuan Wang (CECCM), Hu Zhou (Shanghai Institute of Materia Medica, CAS), Professor Jia Fan (Zhongshan Hospital), the animal and cell-analysis platforms of CECCM, the mass-spectrometry platform of the CAS Center for Excellence in Molecular Plant Sciences, and collaborators at Huazhong Agricultural University. Funding was provided by the National Key R&D Program of China, the CAS Strategic Priority Research Program, the National Natural Science Foundation of China, Shanghai Major Science and Technology Projects, the National Science and Technology Major Project, and the China Postdoctoral Science Foundation.
Article link: https://www.nature.com/articles/s41586-025-09518-6
Cancer immunotherapy—especially approaches that harness T cells to recognize and eliminate malignant cells—has become a major breakthrough in oncology. However, its broad application faces two major obstacles. First, many tumor-associated antigens are expressed at low density on cancer-cell surfaces, making it difficult to effectively activate T cells. Second, these antigens are often expressed at low levels in normal tissues as well, leading to poor specificity and potential off-target toxicity. Thus, strategies capable of strengthening antigen signals while precisely targeting tumor cells are urgently needed.
To tackle this challenge, the research team approached it from a chemical-biology perspective, asking whether proximity-labeling—originally developed as a technique to detect spatially adjacent proteins—could be transformed into a functional regulatory tool, capable of directly amplifying targeting signals on tumor-cell surfaces and thereby “labeling” the cells that should be eliminated by the immune system.
In this study, the authors applied proximity labeling to immune regulation for the first time and developed a completely new strategy for engineering cell-surface proteins, termed Proximity Amplification and Tagging of Cytotoxic Haptens (PATCH). At the center of this strategy is an engineered nanoenzyme (PCN) that can be selectively activated by red light or ultrasound. The nanoenzyme is first delivered to tumor-cell surfaces, and then precisely and non-invasively activated via external red light or ultrasound. Once activated, it catalyzes probes containing an artificial antigen (FITC) to rapidly and massively form covalent bonds with cell-surface proteins within a few nanometers of the nanoenzyme. This process effectively “plants” high-density artificial antigen clusters on the surface of target tumor cells.

These in situ constructed antigen clusters act as “super beacons” for immune cells. Using a bispecific T-cell engager (BiTE) that binds both FITC and CD3 on T cells, the antigen clusters efficiently recruit and cluster T-cell receptors (TCRs), strongly activating T cells and drastically boosting their ability to recognize and kill tumor cells.
The PATCH strategy produced striking therapeutic effects across multiple solid-tumor animal models and patient-derived tumor samples. The study showed that PATCH not only eradicated treated tumors completely, but—importantly—the robust tumor-killing process released abundant tumor antigens, which in turn triggered systemic immune responses against distant, untreated tumors (the “abscopal effect”). It also generated durable immune memory, effectively preventing tumor recurrence.
This work is the first to expand proximity-labeling chemistry from a tool for detecting molecular interactions into a powerful functional regulatory technology. Its key strengths include solving the problem of insufficient natural antigen density through catalytic amplification, and ensuring high therapeutic specificity through precise physical control (light or ultrasound activation). This significantly broadens the landscape of targetable tumor antigens and provides a new conceptual and technological foundation for next-generation immunotherapies that are precise, potent, and low-toxicity.
Professor Shuo Han (CAS Center for Excellence in Molecular Cell Science) and Professor Qiang Gao (Zhongshan Hospital, Fudan University) are co-corresponding authors of the paper. The co-first authors are postdoctoral fellow Shuojun Li (CECCM), PhD student Yinghui Men (Shanghai Jiao Tong University), PhD student Zihan Wang (CECCM), and PhD student Yingcheng Wu (Fudan University). The study was supported by researchers Chenqi Xu and Guangchuan Wang (CECCM), Hu Zhou (Shanghai Institute of Materia Medica, CAS), Professor Jia Fan (Zhongshan Hospital), the animal and cell-analysis platforms of CECCM, the mass-spectrometry platform of the CAS Center for Excellence in Molecular Plant Sciences, and collaborators at Huazhong Agricultural University. Funding was provided by the National Key R&D Program of China, the CAS Strategic Priority Research Program, the National Natural Science Foundation of China, Shanghai Major Science and Technology Projects, the National Science and Technology Major Project, and the China Postdoctoral Science Foundation.
Article link: https://www.nature.com/articles/s41586-025-09518-6


