Xuetao Cao’s Team Reveals A Novel Approach to Improve Immune Therapy via Inhibiting Tumor Cell Macropinocytosis
Source:Yujia Wang
2025-08-26
On August 14, 2025, a research team led by Professor Xuetao Cao from Institute of Basic Medical Sciences, Peking Union Medical College, Chinese Academy of Medical Sciences, published a study titled "Inhibition of tumor cell macropinocytosis driver DHODH reverses immunosuppression and overcomes anti-PD1 resistance" in the top-tier immunology journal Immunity.
Sustained tumor growth requires a continuous supply of nutrients that support energy production and macromolecular synthesis. Tumor cells frequently employ nutrient-scavenging pathways, such as macropinocytosis, to survive and proliferate. Macropinocytosis is a clathrin-independent endocytic process that mediates non-selective uptake of extracellular fluid, proteins, sugars and lipids. However, the metabolic regulation of cancer macropinocytosis in immune escape and its effect on immunotherapy remain unclear.
To identify metabolic molecules mediating tumor cell macropinocytosis, the researchers conducted a metabolism-modulating chemical library screening using a high-content cell imaging system. Combined with the results obtained from a genome-wide CRISPR-Cas9 screening, the team identified dihydroorotate dehydrogenase (DHODH) as an essential driver of tumor cell macropinocytosis both in vitro and in vivo.
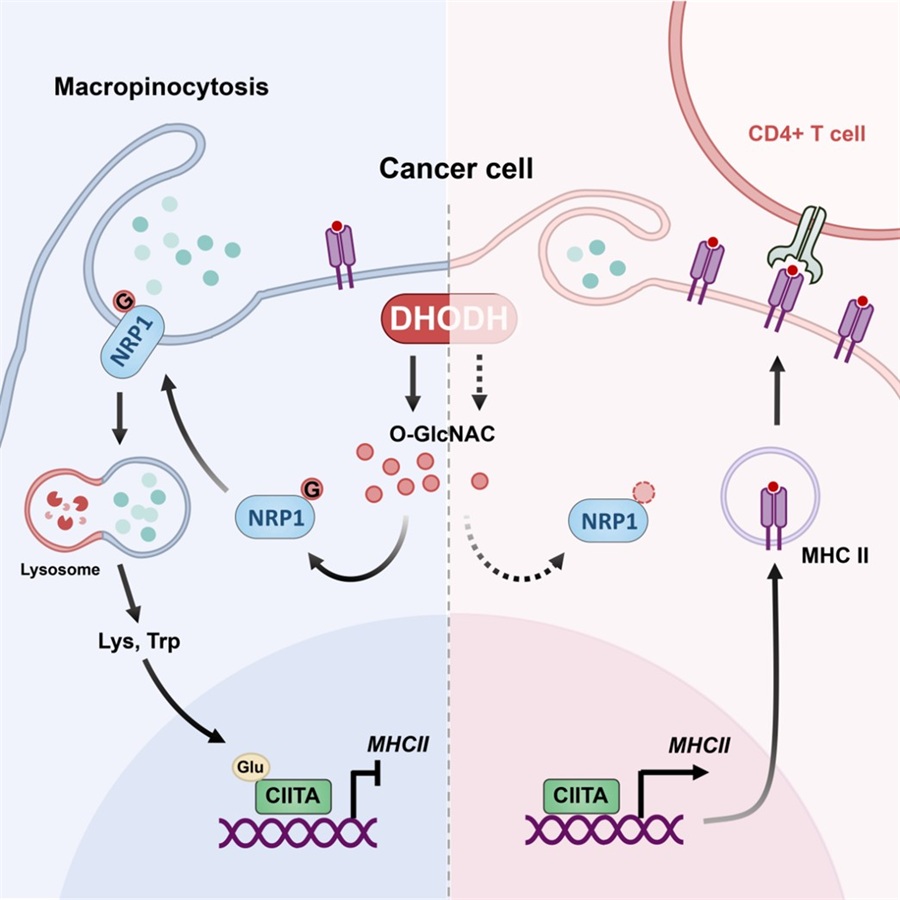
DHODH plays a crucial role in the de novo synthesis of pyrimidine, which metabolic pathway provides the direct sugar donor for protein O-GlcNAc modification. DHODH sustained O-GlcNAcylation of the macropinocytic mediator neuropilin-1 (NRP1) and its membrane localization, mediating tumor cell macropinocytosis. Moreover, the DHODH-NRP1 axis-driven macropinocytosis increased intracellular amounts of lysine and tryptophan, which promoted glutarylation of the transcription factor class II transactivator (CIITA) to repress cancer cell major histocompatibility complex class II (MHC class II) expression. Pharmacological inhibition or genetic deletion of tumor cell-expressed DHODH potently recruited more immune cell infiltration and activated antitumor immunity in vivo, overcoming anti-programmed cell death protein 1 (PD1) resistance. High expression of DHODH and NRP1 in human breast and lung cancer tissues predicted patients’ poor prognosis, while CD74 expression in tumor tissues was higher in the responder groups of immune checkpoint blockade treatment. Therefore, targeting DHODH to inhibit tumor cell macropinocytosis provides a potential approach to reverse immunosuppression for improving cancer immunotherapy.
This work was supported by grants from the National Natural Science Foundation of China, the China National Postdoctoral Program for Innovative Talents, the China Postdoctoral Science Foundation, and the CAMS Innovation Fund for Medical Sciences. Professor Xuetao Cao is the corresponding author, Yujia Wang (Assistant Researcher) serves as the first author.
Article link: https://www.sciencedirect.com/science/article/pii/S107476132500322X
Sustained tumor growth requires a continuous supply of nutrients that support energy production and macromolecular synthesis. Tumor cells frequently employ nutrient-scavenging pathways, such as macropinocytosis, to survive and proliferate. Macropinocytosis is a clathrin-independent endocytic process that mediates non-selective uptake of extracellular fluid, proteins, sugars and lipids. However, the metabolic regulation of cancer macropinocytosis in immune escape and its effect on immunotherapy remain unclear.
To identify metabolic molecules mediating tumor cell macropinocytosis, the researchers conducted a metabolism-modulating chemical library screening using a high-content cell imaging system. Combined with the results obtained from a genome-wide CRISPR-Cas9 screening, the team identified dihydroorotate dehydrogenase (DHODH) as an essential driver of tumor cell macropinocytosis both in vitro and in vivo.
DHODH plays a crucial role in the de novo synthesis of pyrimidine, which metabolic pathway provides the direct sugar donor for protein O-GlcNAc modification. DHODH sustained O-GlcNAcylation of the macropinocytic mediator neuropilin-1 (NRP1) and its membrane localization, mediating tumor cell macropinocytosis. Moreover, the DHODH-NRP1 axis-driven macropinocytosis increased intracellular amounts of lysine and tryptophan, which promoted glutarylation of the transcription factor class II transactivator (CIITA) to repress cancer cell major histocompatibility complex class II (MHC class II) expression. Pharmacological inhibition or genetic deletion of tumor cell-expressed DHODH potently recruited more immune cell infiltration and activated antitumor immunity in vivo, overcoming anti-programmed cell death protein 1 (PD1) resistance. High expression of DHODH and NRP1 in human breast and lung cancer tissues predicted patients’ poor prognosis, while CD74 expression in tumor tissues was higher in the responder groups of immune checkpoint blockade treatment. Therefore, targeting DHODH to inhibit tumor cell macropinocytosis provides a potential approach to reverse immunosuppression for improving cancer immunotherapy.

Figure. Mechanistic model of tumor cell macropinocytosis driver DHODH contributes to immunosuppression
This work was supported by grants from the National Natural Science Foundation of China, the China National Postdoctoral Program for Innovative Talents, the China Postdoctoral Science Foundation, and the CAMS Innovation Fund for Medical Sciences. Professor Xuetao Cao is the corresponding author, Yujia Wang (Assistant Researcher) serves as the first author.
Article link: https://www.sciencedirect.com/science/article/pii/S107476132500322X


