Yichuan Xiao’s Team Reveals a Novel Mechanism of Neutrophil Senescence Influencing Sexual Dimorphism in Tumors
Source:Zhu Qingchen
2025-05-23
Clinical statistics indicate that nearly all non-reproductive system tumors exhibit sexual dimorphism, with males showing significantly higher incidence and mortality rates than females. Although sex hormones (e.g., androgens), sex chromosomes, and related genes are considered potential contributors, the immunological mechanisms underlying tumor sexual dimorphism remain unclear. Tumor-infiltrating immune cells in the tumor microenvironment (TME) play critical roles in regulating tumor immunity and progression. Among them, neutrophils exhibit dual pro- and anti-tumor effects, yet whether their functional heterogeneity contributes to sexual dimorphism in tumors remains unknown.
On April 11, 2025, a research team led by Prof. Xiao Yichuan from the Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences, published a study titled "Microbiota-shaped neutrophil senescence regulates sexual dimorphism in bladder cancer" in Nature Immunology. Combining multi-omics technologies and animal models, this study systematically revealed the pivotal role of gut microbiota-modulated neutrophil senescence in regulating sexual dimorphism in solid tumors, providing a theoretical foundation and molecular targets for sex-specific cancer therapy.
First, the team employed single-cell RNA sequencing to analyze tumor-infiltrating immune cells in male and female bladder cancer-bearing mice. They identified a striking sexual disparity in neutrophil infiltration and discovered an age-associated senescent-like neutrophil subset. Compared to non-senescent neutrophils, these cells exhibited stronger immunosuppressive activity and were enriched in male TMEs, leading to impaired anti-tumor immunity. Based on their unique gene expression profile, this subset was termed Retnlg+Lcn2+ senescence-like neutrophils (RLSNs). Analysis of TCGA data revealed that the presence of RLSNs (identified via signature gene sets) correlated with poorer overall survival (OS) in bladder cancer patients. Depleting RLSNs using the Retnlg-DTR system abolished sex differences in tumor growth, confirming their role in tumor sexual dimorphism.
Given prior evidence that neutrophil senescence is regulated by gut microbiota, the team further found that female mice harbored higher levels of Alistipes shahii, a gut bacterium whose associated metabolite, lurasidone, was more abundant in females. Lurasidone directly targeted LCN2, a hallmark protein of RLSNs. As LCN2 is an iron-chelating protein, lurasidone triggered intracellular Fe²⁺ release, promoting ferroptosis in RLSNs and thereby eliminating their accumulation in female tumors. In contrast, males lacked sufficient A. shahii and lurasidone, leading to suppressed RLSN ferroptosis and their subsequent accumulation in the TME, where they inhibited anti-tumor immunity. Moreover, male-accumulated RLSNs secreted excessive LCN2, which further depleted iron-sensitive A. shahii, creating a vicious cycle.
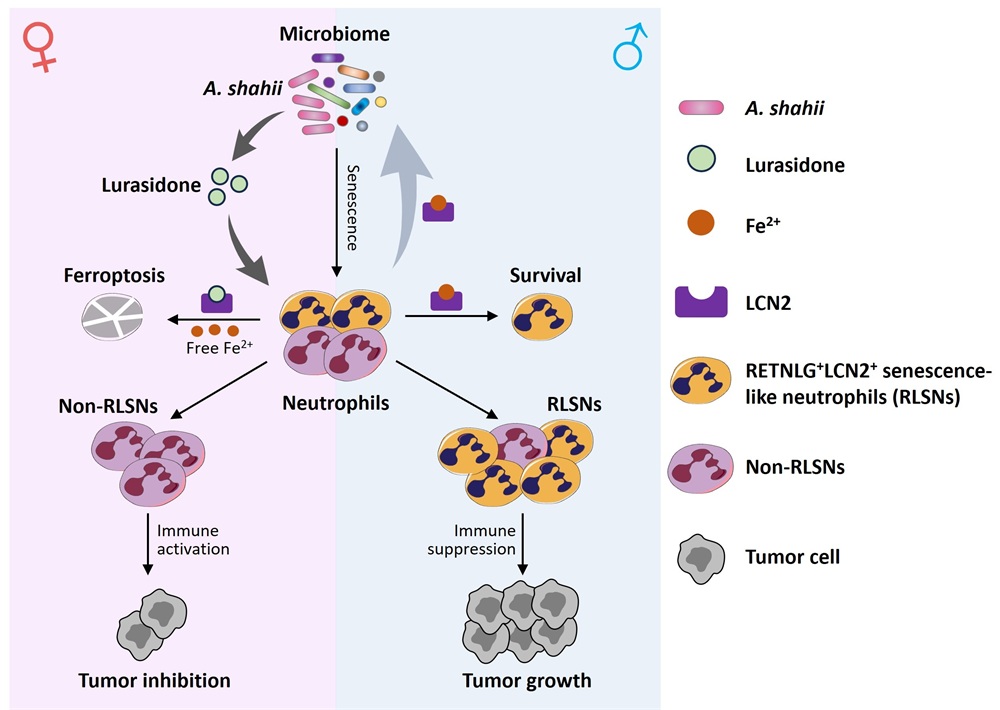
In summary, this study unveils a novel mechanism by which A. shahii-derived lurasidone targets LCN2 to induce RLSN ferroptosis, thereby modulating tumor sexual dimorphism. These findings provide a theoretical basis and molecular targets for sex-tailored therapies in bladder cancer.
Notably, lurasidone, an FDA-approved antipsychotic drug, may be repurposed to enhance anti-tumor immunity—either alone or in combination with existing immunotherapies—opening new avenues for sex-specific bladder cancer treatment.
The study was co-corresponding authored by Prof. Xiao Yichuan and Prof. Qin Jun from the Shanghai Institute of Nutrition and Health, CAS, and Prof. Liu Chenli from the Shenzhen Institute of Advanced Technology, CAS. Co-first authors included Dr. Zhu Qingchen and Dr. Huang Huan, Zhang Guiheng (Shanghai Institute of Nutrition and Health, CAS), and Dr. Cao Ming (Renji Hospital, Shanghai Jiao Tong University School of Medicine). The work was supported by grants from the Ministry of Science and Technology, the National Natural Science Foundation of China, and the Chinese Academy of Sciences, with additional support from the core facilities at the Shanghai Institute of Nutrition and Health, CAS.
Original artical: https://www.nature.com/articles/s41590-025-02126-6
On April 11, 2025, a research team led by Prof. Xiao Yichuan from the Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences, published a study titled "Microbiota-shaped neutrophil senescence regulates sexual dimorphism in bladder cancer" in Nature Immunology. Combining multi-omics technologies and animal models, this study systematically revealed the pivotal role of gut microbiota-modulated neutrophil senescence in regulating sexual dimorphism in solid tumors, providing a theoretical foundation and molecular targets for sex-specific cancer therapy.
First, the team employed single-cell RNA sequencing to analyze tumor-infiltrating immune cells in male and female bladder cancer-bearing mice. They identified a striking sexual disparity in neutrophil infiltration and discovered an age-associated senescent-like neutrophil subset. Compared to non-senescent neutrophils, these cells exhibited stronger immunosuppressive activity and were enriched in male TMEs, leading to impaired anti-tumor immunity. Based on their unique gene expression profile, this subset was termed Retnlg+Lcn2+ senescence-like neutrophils (RLSNs). Analysis of TCGA data revealed that the presence of RLSNs (identified via signature gene sets) correlated with poorer overall survival (OS) in bladder cancer patients. Depleting RLSNs using the Retnlg-DTR system abolished sex differences in tumor growth, confirming their role in tumor sexual dimorphism.
Given prior evidence that neutrophil senescence is regulated by gut microbiota, the team further found that female mice harbored higher levels of Alistipes shahii, a gut bacterium whose associated metabolite, lurasidone, was more abundant in females. Lurasidone directly targeted LCN2, a hallmark protein of RLSNs. As LCN2 is an iron-chelating protein, lurasidone triggered intracellular Fe²⁺ release, promoting ferroptosis in RLSNs and thereby eliminating their accumulation in female tumors. In contrast, males lacked sufficient A. shahii and lurasidone, leading to suppressed RLSN ferroptosis and their subsequent accumulation in the TME, where they inhibited anti-tumor immunity. Moreover, male-accumulated RLSNs secreted excessive LCN2, which further depleted iron-sensitive A. shahii, creating a vicious cycle.

Mechanistic model of neutrophil senescence-mediated tumor sexual dimorphism
In summary, this study unveils a novel mechanism by which A. shahii-derived lurasidone targets LCN2 to induce RLSN ferroptosis, thereby modulating tumor sexual dimorphism. These findings provide a theoretical basis and molecular targets for sex-tailored therapies in bladder cancer.
Notably, lurasidone, an FDA-approved antipsychotic drug, may be repurposed to enhance anti-tumor immunity—either alone or in combination with existing immunotherapies—opening new avenues for sex-specific bladder cancer treatment.
The study was co-corresponding authored by Prof. Xiao Yichuan and Prof. Qin Jun from the Shanghai Institute of Nutrition and Health, CAS, and Prof. Liu Chenli from the Shenzhen Institute of Advanced Technology, CAS. Co-first authors included Dr. Zhu Qingchen and Dr. Huang Huan, Zhang Guiheng (Shanghai Institute of Nutrition and Health, CAS), and Dr. Cao Ming (Renji Hospital, Shanghai Jiao Tong University School of Medicine). The work was supported by grants from the Ministry of Science and Technology, the National Natural Science Foundation of China, and the Chinese Academy of Sciences, with additional support from the core facilities at the Shanghai Institute of Nutrition and Health, CAS.
Original artical: https://www.nature.com/articles/s41590-025-02126-6


