Dr. Ji Wang's Group Develops a Novel Vaccine Adjuvant to Overcome the "Last Mile" Challenge in Antigen Cross-Presentation
Source:Juan Jiang
2025-05-22
Vaccines are the most economical and effective measures for preventing and treating infectious diseases, and also one of the most promising therapeutic approaches for chronic conditions such as cancer. Current prophylactic vaccines are not sufficiently effective against highly mutable pathogens, while therapeutic vaccines cannot yet eradicate chronic infections and tumors. Overcoming the common underlying immunological challenges is the wellspring of vaccine technology innovation. CD8+ T cells are the primary effector cells for clearing infected cells and tumor cells, often targeting conserved epitopes. Their efficient induction has long been a goal for broadly protective prophylactic and effective therapeutic vaccines but has been constrained by the efficiency bottleneck of antigen cross-presentation.
Antigen cross-presentation primarily occurs in dendritic cells (DCs) and involves a process where antigens are taken up into the cytoplasm, reach the endoplasmic reticulum (ER), and are subsequently processed and loaded onto MHC-I molecules. Previous research has mainly focused on designing adjuvants and delivery systems to deliver antigens into the DC cytoplasm, activate their innate immune pathways, and promote the expression of co-stimulatory molecules and cytokines. However, even with potent adjuvants, the induction of CD8+ T cell immune responses often falls short of expectations. A recent literature review summarized that tumor peptide vaccines in clinical studies typically induce antigen-specific CD8+ T cells accounting for only 0.1% of total CD8+ T cells in the blood. This is far below the 10-20% level achievable by live virus vaccines (such as yellow fever or smallpox) that can induce lifelong protection (Nat Rev Immunol. 2024 Mar;24(3):213-227).
Thanks to decades of effort by numerous research teams, current vaccine technology has excelled in antigen uptake and activation of innate immune pathways. To further bridge the significant gap that remains compared to live virus vaccines, it is necessary to revisit the immunological process of cross-presentation and find the missing link. How to guide antigens that have entered the cytoplasm to the ER, thereby completing this "last mile" of delivery, may be the key. However, the field has long lacked a high-affinity ER-targeting molecule capable of accomplishing this final delivery step.
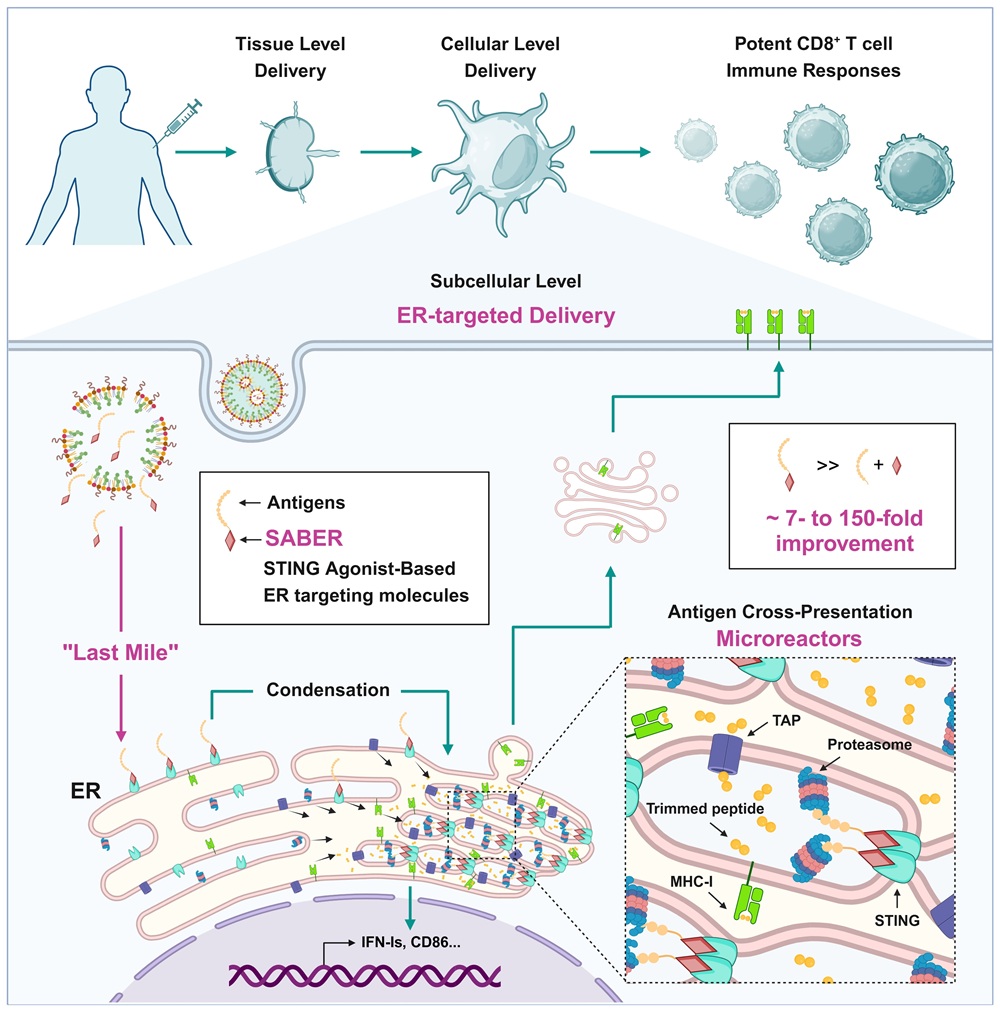
Dr. Ji Wang's group from the First Affiliated Hospital of Sun Yat-sen University, in collaboration with Dr. Lu Lu's team from Fudan University and Yingyue Zeng's team from Liaoning University, published a research paper titled "STING agonist-based ER-targeting molecules boost antigen cross-presentation" in the May 1, 2025 issue of Nature. They developed novel small molecules with high affinity for ER targeting, revealing the critical role of this "last mile" antigen delivery to the ER for cross-presentation. Based on this, they pioneered a new-concept vaccine adjuvant with dual functions: antigen ER targeting and immune activation, significantly enhancing the induction of CD8+ T cell immune responses. Nature concurrently published a News & Views article titled "Crucial meeting: molecule helps vaccine to interact with killer T cells."
Drawing on over a decade of experience in vaccine adjuvants and the STING field, the authors proposed that the STING protein, located on the ER, could be the key breakthrough for achieving ER targeting. However, they faced multiple challenges in molecular design, antigen conjugation strategy, and vaccine formulation technology. Through a combination of systematic screening and rational design, the authors screened and designed dozens of STING agonists and their antigen linkers, ultimately obtaining the SABER (STING Agonist-Based ER-targeting) molecule. This molecule can both target antigens to the ER and maintain highly efficient STING activation. Subsequently, the authors screened and designed a compatible lipid nanoparticle (LNP) delivery system and microfluidic packaging technology, ultimately formulating the SABER-antigen complex into a highly efficient and stable vaccine that can be stored stably at 4°C for over 140 days.
Using techniques such as immunofluorescence, ER isolation, and proximity labeling, the authors confirmed from multiple perspectives that SABER can effectively target antigens to the ER. They also showed that SABER promotes the oligomerization of STING, which folds and compacts the ER membrane, concentrating antigens and key components of cross-presentation (such as TAP and the proteasome) to form an efficient cross-presentation "microreactor," thereby enhancing antigen processing and transport efficiency. Ultimately, SABER can significantly increase the number of MHC-I-peptide complexes on the DC surface and the proliferation of antigen-specific CD8+ T cells.
To delve deeper into the underlying mechanisms, the authors designed a SABER control molecule containing a disulfide bond, allowing for intracellular cleavage from the antigen. This cleavable SABER molecule's ability to activate STING was unaffected, but its capacity to enhance cross-presentation was significantly reduced, demonstrating that "ER-targeted delivery of the antigen" is the core mechanism of SABER. Furthermore, using in vitro multi-subset DC cultures, in vivo DC subset analysis, and Batf3-/- mice, the authors found that SABER is effective for both cDC1 and cDC2 subsets and can drive cross-presentation in various antigen-presenting cells in vivo.
Subsequent vaccination experiments revealed that SABER's ability to enhance CD8+ T cell immune response induction not only far surpassed the potent STING agonist diABZI but was also more than 10-fold higher than control adjuvants like Poly I:C, ODN1018, and ISCOMs (after three immunizations). These adjuvants have all been reported to effectively promote the induction of CD8+ T cell immune responses. Among them, the TLR3 agonist Poly I:C is widely used in clinical research for tumor neoantigen vaccines, the TLR9 agonist ODN1018 is an adjuvant in a commercial hepatitis B vaccine, and the ISCOMs family is used in malaria vaccines and SARS-CoV-2 subunit vaccines. In mouse models, these control adjuvants could increase the proportion of antigen-specific CD8+ T cells in the blood to 0.5%-3%, whereas SABER achieved 30%.
Harnessing its outstanding ability to induce CD8+ T cell immune responses, tumor neoantigen vaccines and viral vaccines based on SABER technology have shown excellent preventive and therapeutic effects in various animal models of cancer and infectious diseases. For example, in a mouse melanoma B16F10 model resistant to immune checkpoint inhibitor therapy, a vaccine prepared with SABER technology targeting a single neoantigen epitope was sufficient to effectively synergize with PD-1 monoclonal antibody treatment to inhibit tumor growth, an effect significantly superior to Poly I:C, which is widely used in clinical studies. Furthermore, a SARS-CoV-2 peptide vaccine based on SABER technology substantially enhanced CD8+ T cell responses against conserved epitopes, reducing viral loads by 100-fold after challenge with a mutated strain. In addition, SABER not only enhances cellular immunity but also possesses an ability to augment humoral immune responses comparable to or even greater than existing adjuvants. When used with subunit vaccines, it can effectively enhance the induction of cross-protective neutralizing antibodies.
Advanced adjuvants and delivery systems are a hallmark of new-generation vaccines and are essential for developing next-generation prophylactic vaccines with broad-spectrum protection against infectious diseases, as well as therapeutic vaccines capable of eradicating chronic viral infections and tumors. Dr. Ji Wang's group has long been engaged in vaccine research, establishing an immunoengineering research system to explore the fundamental immunological questions behind adjuvants and delivery systems, develop tools to study these questions, and find solutions. In 2020, the team published a research article in Science reporting key factors in the immune microenvironment that regulate DC cross-presentation and CD8+ T cell immune response induction. Now, they have further advanced the regulation of cross-presentation to the subcellular level. This series of studies not only provides an innovative solution to the long-standing scientific challenge of inducing CD8+ T cell immunity but also offers a critical, common foundational technology for the development of next-generation vaccines.
Dr. Ji Wang from the First Affiliated Hospital of Sun Yat-sen University is the lead corresponding author. Dr. Lu Lu from Fudan University and Dr. Yingyue Zeng from Liaoning University are co-corresponding authors. Dr. Xiafeng Wang, Zhangping Huang, Dr. Liru Shang, and Dr. Juan Jiang from the First Affiliated Hospital of Sun Yat-sen University, along with Dr. Lixiao Xing from Fudan University, are co-first authors of this paper. This research was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Liaoning Provincial Department of Education Scientific Research Project.
Original article: https://www.nature.com/articles/s41586-025-08758-w
Antigen cross-presentation primarily occurs in dendritic cells (DCs) and involves a process where antigens are taken up into the cytoplasm, reach the endoplasmic reticulum (ER), and are subsequently processed and loaded onto MHC-I molecules. Previous research has mainly focused on designing adjuvants and delivery systems to deliver antigens into the DC cytoplasm, activate their innate immune pathways, and promote the expression of co-stimulatory molecules and cytokines. However, even with potent adjuvants, the induction of CD8+ T cell immune responses often falls short of expectations. A recent literature review summarized that tumor peptide vaccines in clinical studies typically induce antigen-specific CD8+ T cells accounting for only 0.1% of total CD8+ T cells in the blood. This is far below the 10-20% level achievable by live virus vaccines (such as yellow fever or smallpox) that can induce lifelong protection (Nat Rev Immunol. 2024 Mar;24(3):213-227).
Thanks to decades of effort by numerous research teams, current vaccine technology has excelled in antigen uptake and activation of innate immune pathways. To further bridge the significant gap that remains compared to live virus vaccines, it is necessary to revisit the immunological process of cross-presentation and find the missing link. How to guide antigens that have entered the cytoplasm to the ER, thereby completing this "last mile" of delivery, may be the key. However, the field has long lacked a high-affinity ER-targeting molecule capable of accomplishing this final delivery step.

Dr. Ji Wang's group from the First Affiliated Hospital of Sun Yat-sen University, in collaboration with Dr. Lu Lu's team from Fudan University and Yingyue Zeng's team from Liaoning University, published a research paper titled "STING agonist-based ER-targeting molecules boost antigen cross-presentation" in the May 1, 2025 issue of Nature. They developed novel small molecules with high affinity for ER targeting, revealing the critical role of this "last mile" antigen delivery to the ER for cross-presentation. Based on this, they pioneered a new-concept vaccine adjuvant with dual functions: antigen ER targeting and immune activation, significantly enhancing the induction of CD8+ T cell immune responses. Nature concurrently published a News & Views article titled "Crucial meeting: molecule helps vaccine to interact with killer T cells."
Drawing on over a decade of experience in vaccine adjuvants and the STING field, the authors proposed that the STING protein, located on the ER, could be the key breakthrough for achieving ER targeting. However, they faced multiple challenges in molecular design, antigen conjugation strategy, and vaccine formulation technology. Through a combination of systematic screening and rational design, the authors screened and designed dozens of STING agonists and their antigen linkers, ultimately obtaining the SABER (STING Agonist-Based ER-targeting) molecule. This molecule can both target antigens to the ER and maintain highly efficient STING activation. Subsequently, the authors screened and designed a compatible lipid nanoparticle (LNP) delivery system and microfluidic packaging technology, ultimately formulating the SABER-antigen complex into a highly efficient and stable vaccine that can be stored stably at 4°C for over 140 days.
Using techniques such as immunofluorescence, ER isolation, and proximity labeling, the authors confirmed from multiple perspectives that SABER can effectively target antigens to the ER. They also showed that SABER promotes the oligomerization of STING, which folds and compacts the ER membrane, concentrating antigens and key components of cross-presentation (such as TAP and the proteasome) to form an efficient cross-presentation "microreactor," thereby enhancing antigen processing and transport efficiency. Ultimately, SABER can significantly increase the number of MHC-I-peptide complexes on the DC surface and the proliferation of antigen-specific CD8+ T cells.
To delve deeper into the underlying mechanisms, the authors designed a SABER control molecule containing a disulfide bond, allowing for intracellular cleavage from the antigen. This cleavable SABER molecule's ability to activate STING was unaffected, but its capacity to enhance cross-presentation was significantly reduced, demonstrating that "ER-targeted delivery of the antigen" is the core mechanism of SABER. Furthermore, using in vitro multi-subset DC cultures, in vivo DC subset analysis, and Batf3-/- mice, the authors found that SABER is effective for both cDC1 and cDC2 subsets and can drive cross-presentation in various antigen-presenting cells in vivo.
Subsequent vaccination experiments revealed that SABER's ability to enhance CD8+ T cell immune response induction not only far surpassed the potent STING agonist diABZI but was also more than 10-fold higher than control adjuvants like Poly I:C, ODN1018, and ISCOMs (after three immunizations). These adjuvants have all been reported to effectively promote the induction of CD8+ T cell immune responses. Among them, the TLR3 agonist Poly I:C is widely used in clinical research for tumor neoantigen vaccines, the TLR9 agonist ODN1018 is an adjuvant in a commercial hepatitis B vaccine, and the ISCOMs family is used in malaria vaccines and SARS-CoV-2 subunit vaccines. In mouse models, these control adjuvants could increase the proportion of antigen-specific CD8+ T cells in the blood to 0.5%-3%, whereas SABER achieved 30%.
Harnessing its outstanding ability to induce CD8+ T cell immune responses, tumor neoantigen vaccines and viral vaccines based on SABER technology have shown excellent preventive and therapeutic effects in various animal models of cancer and infectious diseases. For example, in a mouse melanoma B16F10 model resistant to immune checkpoint inhibitor therapy, a vaccine prepared with SABER technology targeting a single neoantigen epitope was sufficient to effectively synergize with PD-1 monoclonal antibody treatment to inhibit tumor growth, an effect significantly superior to Poly I:C, which is widely used in clinical studies. Furthermore, a SARS-CoV-2 peptide vaccine based on SABER technology substantially enhanced CD8+ T cell responses against conserved epitopes, reducing viral loads by 100-fold after challenge with a mutated strain. In addition, SABER not only enhances cellular immunity but also possesses an ability to augment humoral immune responses comparable to or even greater than existing adjuvants. When used with subunit vaccines, it can effectively enhance the induction of cross-protective neutralizing antibodies.
Advanced adjuvants and delivery systems are a hallmark of new-generation vaccines and are essential for developing next-generation prophylactic vaccines with broad-spectrum protection against infectious diseases, as well as therapeutic vaccines capable of eradicating chronic viral infections and tumors. Dr. Ji Wang's group has long been engaged in vaccine research, establishing an immunoengineering research system to explore the fundamental immunological questions behind adjuvants and delivery systems, develop tools to study these questions, and find solutions. In 2020, the team published a research article in Science reporting key factors in the immune microenvironment that regulate DC cross-presentation and CD8+ T cell immune response induction. Now, they have further advanced the regulation of cross-presentation to the subcellular level. This series of studies not only provides an innovative solution to the long-standing scientific challenge of inducing CD8+ T cell immunity but also offers a critical, common foundational technology for the development of next-generation vaccines.
Dr. Ji Wang from the First Affiliated Hospital of Sun Yat-sen University is the lead corresponding author. Dr. Lu Lu from Fudan University and Dr. Yingyue Zeng from Liaoning University are co-corresponding authors. Dr. Xiafeng Wang, Zhangping Huang, Dr. Liru Shang, and Dr. Juan Jiang from the First Affiliated Hospital of Sun Yat-sen University, along with Dr. Lixiao Xing from Fudan University, are co-first authors of this paper. This research was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Liaoning Provincial Department of Education Scientific Research Project.
Original article: https://www.nature.com/articles/s41586-025-08758-w


