Protective SARS-CoV-2-Specific T Cells Take Up Residence in the Lungs of COVID-19 Patients
Source:Airu Zhu
2025-04-01
The COVID-19 pandemic has posed a significant global public health challenge. While vaccines have played a crucial role in controlling the spread of the virus, breakthrough infections continue to occur. Moreover, with a large population base, China faces particular challenges in protecting vulnerable groups such as elderly individuals with underlying conditions and immunocompromised patients, including those with cancer. These populations have lower vaccination rates and are at higher risk of severe disease upon SARS-CoV-2 infection, making effective clinical management a persistent challenge. Understanding the immune response dynamics in COVID-19, particularly in the primary site of infection—the lungs—is critical for developing effective therapeutic strategies. However, despite extensive research on immune responses to SARS-CoV-2, the characteristics and function of virus-specific T cells in the lungs have remained insufficiently explored.
On January 28, 2025, a research team led by Professor Jin-Cun Zhao, Professor Jing-Xian Zhao, Academician Nan-Shan Zhong, Professor Yong-Hao Xu, and Professor Xiao-Bo Chen from the First Affiliated Hospital of Guangzhou Medical University/Guangzhou National Laboratory published a study in Nature Immunology titled "Robust mucosal SARS-CoV-2-specific T cells effectively combat COVID-19 and establish polyfunctional resident memory in patient lungs." This study systematically elucidates the activation characteristics and protective function of SARS-CoV-2-specific T cells in the lungs using bronchoalveolar lavage fluid (BALF) and paired peripheral blood samples from COVID-19 patients.
The research team analyzed 159 COVID-19 patients, collecting 122 BALF samples and 280 blood samples, including 27 paired BALF and blood samples from 24 individuals. Using state-of-the-art techniques such as single-cell RNA sequencing (scRNA-seq), single-cell T cell receptor (TCR) sequencing, and high-dimensional flow cytometry, the team conducted a comprehensive analysis of SARS-CoV-2-specific T cells in both the respiratory tract and peripheral blood. The findings revealed that SARS-CoV-2-specific T cells in the lungs were highly activated, with levels independent of those in peripheral blood, indicating that peripheral T cell responses do not accurately reflect the immune status in the lungs. Correlation analyses demonstrated that the proportion of SARS-CoV-2-specific T cells in the lungs—but not in peripheral blood—was significantly associated with reduced viral load, decreased systemic inflammation, and improved respiratory function. These findings highlight the pivotal role of mucosal virus-specific T cells in controlling viral replication and mitigating COVID-19 severity.
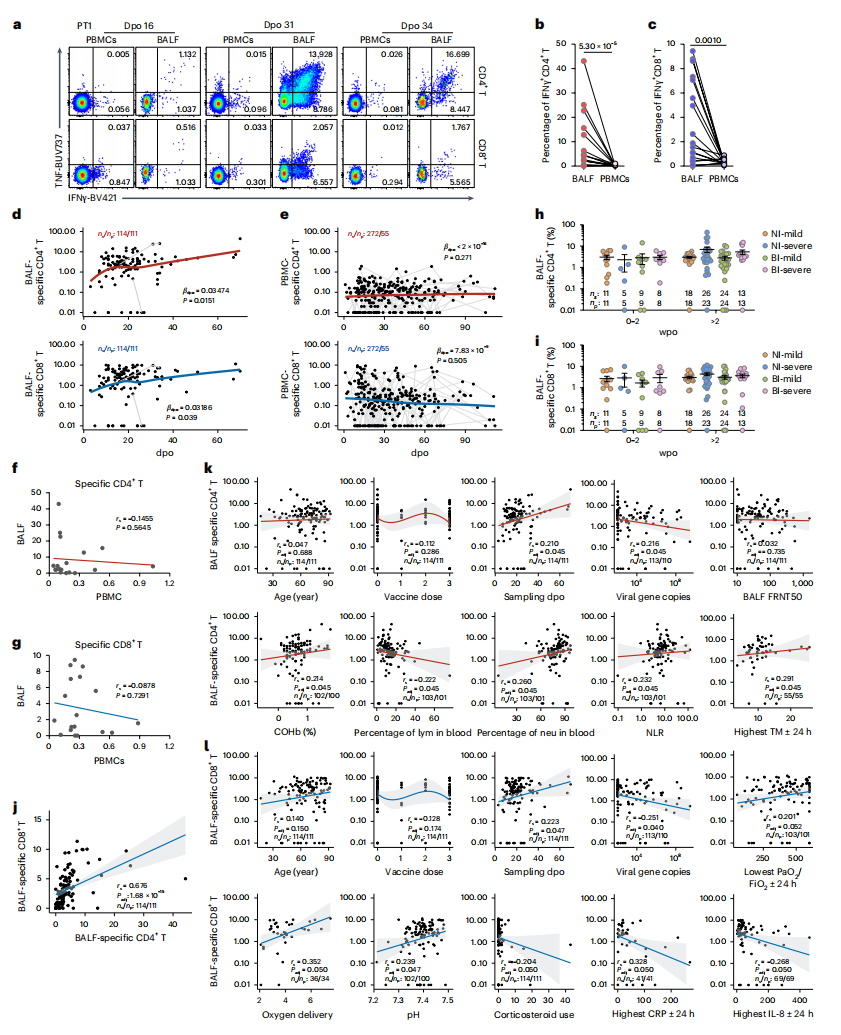
Compared to their peripheral counterparts, SARS-CoV-2-specific T cells in the lungs exhibited stronger activation, proliferation, and multifunctional cytokine secretion capabilities, alongside a distinct glycolytic metabolic profile that supports their effector functions. Transcriptomic analysis further revealed that pathways related to interferon response, T cell activation, inflammation, tissue migration, proliferation, and metabolism were significantly upregulated in these lung-resident virus-specific T cells.
Importantly, after viral clearance, these SARS-CoV-2-specific T cells maintained a polyfunctional resident memory phenotype, enabling rapid responses upon reinfection and providing long-term protection against COVID-19. Notably, the study found no significant difference in the proportion of SARS-CoV-2-specific T cells in the lungs between individuals who had received intramuscular inactivated COVID-19 vaccines and those who were unvaccinated during their first infection. This suggests that intramuscular vaccination may not effectively establish T cell memory in the respiratory tract, underscoring the need for developing mucosal vaccines that can elicit localized airway immunity.
This study provides critical insights into the distinct activation and tissue-resident features of mucosal SARS-CoV-2-specific T cells and their essential role in viral clearance and disease mitigation. These findings offer a scientific foundation for developing more effective mucosal vaccines to combat COVID-19 and other respiratory infections.
Professor Jin-Cun Zhao from the First Affiliated Hospital of Guangzhou Medical University/Guangzhou National Laboratory served as the senior corresponding author, with Professor Jing-Xian Zhao, Academician Nan-Shan Zhong, Professor Yong-Hao Xu, and Professor Xiao-Bo Chen as co-corresponding authors. The study’s co-first authors include postdoctoral fellow Ai-Ru Zhu, PhD candidate Zhao Chen, Professor Qi-Hong Yan, Professor Mei Jiang, Dr. Xue-Song Liu, Dr. Zheng-Tu Li, Dr. Na Li, Dr. Chun-Li Tang, and Dr. Wen-Hua Jian. This research was supported by grants from the Ministry of Science and Technology of China, Guangzhou National Laboratory, the National Key Laboratory of Respiratory Diseases, and the National Natural Science Foundation of China.
Article Link: https://www.nature.com/articles/s41590-024-02072-9
On January 28, 2025, a research team led by Professor Jin-Cun Zhao, Professor Jing-Xian Zhao, Academician Nan-Shan Zhong, Professor Yong-Hao Xu, and Professor Xiao-Bo Chen from the First Affiliated Hospital of Guangzhou Medical University/Guangzhou National Laboratory published a study in Nature Immunology titled "Robust mucosal SARS-CoV-2-specific T cells effectively combat COVID-19 and establish polyfunctional resident memory in patient lungs." This study systematically elucidates the activation characteristics and protective function of SARS-CoV-2-specific T cells in the lungs using bronchoalveolar lavage fluid (BALF) and paired peripheral blood samples from COVID-19 patients.
The research team analyzed 159 COVID-19 patients, collecting 122 BALF samples and 280 blood samples, including 27 paired BALF and blood samples from 24 individuals. Using state-of-the-art techniques such as single-cell RNA sequencing (scRNA-seq), single-cell T cell receptor (TCR) sequencing, and high-dimensional flow cytometry, the team conducted a comprehensive analysis of SARS-CoV-2-specific T cells in both the respiratory tract and peripheral blood. The findings revealed that SARS-CoV-2-specific T cells in the lungs were highly activated, with levels independent of those in peripheral blood, indicating that peripheral T cell responses do not accurately reflect the immune status in the lungs. Correlation analyses demonstrated that the proportion of SARS-CoV-2-specific T cells in the lungs—but not in peripheral blood—was significantly associated with reduced viral load, decreased systemic inflammation, and improved respiratory function. These findings highlight the pivotal role of mucosal virus-specific T cells in controlling viral replication and mitigating COVID-19 severity.

Compared to their peripheral counterparts, SARS-CoV-2-specific T cells in the lungs exhibited stronger activation, proliferation, and multifunctional cytokine secretion capabilities, alongside a distinct glycolytic metabolic profile that supports their effector functions. Transcriptomic analysis further revealed that pathways related to interferon response, T cell activation, inflammation, tissue migration, proliferation, and metabolism were significantly upregulated in these lung-resident virus-specific T cells.
Importantly, after viral clearance, these SARS-CoV-2-specific T cells maintained a polyfunctional resident memory phenotype, enabling rapid responses upon reinfection and providing long-term protection against COVID-19. Notably, the study found no significant difference in the proportion of SARS-CoV-2-specific T cells in the lungs between individuals who had received intramuscular inactivated COVID-19 vaccines and those who were unvaccinated during their first infection. This suggests that intramuscular vaccination may not effectively establish T cell memory in the respiratory tract, underscoring the need for developing mucosal vaccines that can elicit localized airway immunity.
This study provides critical insights into the distinct activation and tissue-resident features of mucosal SARS-CoV-2-specific T cells and their essential role in viral clearance and disease mitigation. These findings offer a scientific foundation for developing more effective mucosal vaccines to combat COVID-19 and other respiratory infections.
Professor Jin-Cun Zhao from the First Affiliated Hospital of Guangzhou Medical University/Guangzhou National Laboratory served as the senior corresponding author, with Professor Jing-Xian Zhao, Academician Nan-Shan Zhong, Professor Yong-Hao Xu, and Professor Xiao-Bo Chen as co-corresponding authors. The study’s co-first authors include postdoctoral fellow Ai-Ru Zhu, PhD candidate Zhao Chen, Professor Qi-Hong Yan, Professor Mei Jiang, Dr. Xue-Song Liu, Dr. Zheng-Tu Li, Dr. Na Li, Dr. Chun-Li Tang, and Dr. Wen-Hua Jian. This research was supported by grants from the Ministry of Science and Technology of China, Guangzhou National Laboratory, the National Key Laboratory of Respiratory Diseases, and the National Natural Science Foundation of China.
Article Link: https://www.nature.com/articles/s41590-024-02072-9


