Dr. Jie Zhou’s Team from Tianjin Medical University Reveals Bile Acid Receptor FXR Exacerbates Necrotizing Enterocolitis by Promoting Epithelial Ferroptosis
Source:Jie Zhou
2025-03-24
Necrotizing enterocolitis (NEC) is a prevalent pediatric emergency characterized by intestinal ischemia and necrosis due to excessive inflammation. As of 2020, the global incidence of NEC is approximately 7% among infants with very low birth weight (<1500g). The long-term mortality rate associated with NEC exceeds 30%, and it can reach up to 80% in cases necessitating immediate surgery. The pathogenesis of NEC is multifactorial, involving genetic predispositions, preterm birth, and inappropriate feeding practices. Current clinical management primarily aims at symptomatic relief, including the cessation of enteral feeding, provision of hemodynamic support, and administration of broad-spectrum antibiotics, as no specific therapies are currently available. Immature intestinal mucosal immunity, dysbiosis, and hyperactivation of inflammatory signaling in the gut epithelium play critical roles in NEC progression. Studies indicate that ileal bile acid levels correlate positively with NEC incidence and severity, and NEC patients exhibit elevated expression of the bile acid receptor FXR. Nonetheless, the mechanisms by which bile acids interact with the microbiota and mucosal immunity in the early infancy, as well as its specific role in the pathogenesis of NEC, remain to be fully elucidated.
On February 28, 2025, the research team led by Dr. Jie Zhou at Tianjin Medical University published a study titled “Bile acid receptor FXR promotes intestinal epithelial ferroptosis and subsequent ILC3 dysfunction in neonatal necrotizing enterocolitis” in Immunity. This study reveals that FXR expression is markedly upregulated in intestinal epithelial cells (IECs) of NEC patients and its mouse model. Elevated FXR promotes IEC ferroptosis by targeting Acsl4. Lipid peroxides released from ferroptotic IECs suppress interleukin-22 (IL-22) secretion by type 3 innate lymphoid cells (ILC3s), exacerbating NEC. Targeted inhibition of intestinal FXR or ferroptosis alleviates NEC in mice.

The authors initially conducted an analysis of clinical neonatal plasma samples, discovering that plasma FGF19, a downstream target of FXR, exhibits an inverse correlation with neonatal maturity and a positive correlation with intestinal permeability and inflammation. Through the application of immunofluorescence staining of intestinal tissues from NEC patients, human fecal transplantation into neonatal mice, and the use of NEC models with IEC-specific FXR knockout mice, they demonstrate that dysbiosis leads to excessive activation of FXR, thereby exacerbating NEC pathology during the early postnatal period.
Mechanistic investigations have demonstrated that FXR facilitates the ferroptosis of IECs by directly targeting Acsl4, a critical mediator of this process. The inhibition of ACSL4 significantly reduced FXR-induced ferroptosis in IECs. Transcriptional analysis of intestinal tissues revealed that the deletion of FXR specifically in IECs led to an upregulation of IL-22-related molecules. Considering that IL-22 is predominantly secreted by ILC3s and promotes the production of Reg3b/Reg3g in epithelial cells to maintain gut homeostasis, they focused on ILC3-IL-22 for further study. Flow cytometry analysis showed that IEC-specific FXR knockout mice displayed higher levels of ILC3 frequencies and IL-22 production. Further in vitro co-culture experiments confirmed that oxidized lipids from ferroptotic IECs suppress IL-22 secretion. The administration of intestinal FXR antagonists, ferroptosis inhibitors, or ACSL4 inhibitors significantly enhanced survival rates and decreased intestinal permeability in NEC models. Finally, clinical observations revealed a positive correlation between the levels of oxidized lipids and the severity of NEC in patients.
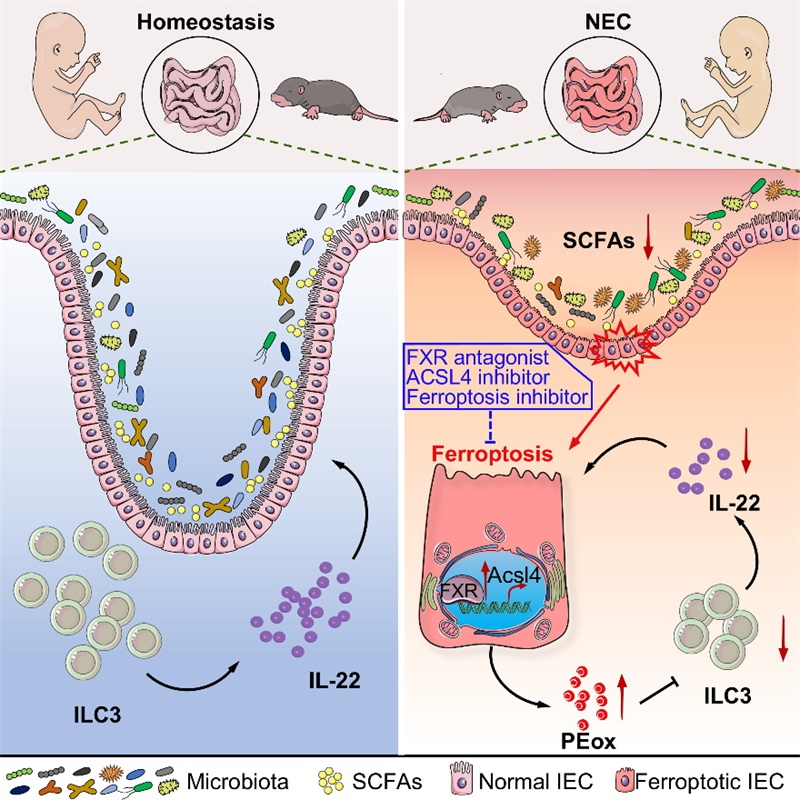
This study collectively offers novel insights into the pathogenesis of NEC by elucidating the complex interactions among microbiota, intestinal epithelium, and immune cells in the intestines during early infancy. It also underscores the therapeutic potential of targeting intestinal FXR and ferroptosis in the treatment of NEC.
Dr. Jie Zhou from Tianjin Medical University/Tianjin Institute of Immunology, is the corresponding author. Team members Ph.D. candidate Yuxin Zhang, Dr. Yuchao Jing, Dr. Juan He, and Ph.D. candidate Rui Dong, share the co-first authorship.
Article Link: https://doi.org/10.1016/j.immuni.2025.02.003
On February 28, 2025, the research team led by Dr. Jie Zhou at Tianjin Medical University published a study titled “Bile acid receptor FXR promotes intestinal epithelial ferroptosis and subsequent ILC3 dysfunction in neonatal necrotizing enterocolitis” in Immunity. This study reveals that FXR expression is markedly upregulated in intestinal epithelial cells (IECs) of NEC patients and its mouse model. Elevated FXR promotes IEC ferroptosis by targeting Acsl4. Lipid peroxides released from ferroptotic IECs suppress interleukin-22 (IL-22) secretion by type 3 innate lymphoid cells (ILC3s), exacerbating NEC. Targeted inhibition of intestinal FXR or ferroptosis alleviates NEC in mice.

The authors initially conducted an analysis of clinical neonatal plasma samples, discovering that plasma FGF19, a downstream target of FXR, exhibits an inverse correlation with neonatal maturity and a positive correlation with intestinal permeability and inflammation. Through the application of immunofluorescence staining of intestinal tissues from NEC patients, human fecal transplantation into neonatal mice, and the use of NEC models with IEC-specific FXR knockout mice, they demonstrate that dysbiosis leads to excessive activation of FXR, thereby exacerbating NEC pathology during the early postnatal period.
Mechanistic investigations have demonstrated that FXR facilitates the ferroptosis of IECs by directly targeting Acsl4, a critical mediator of this process. The inhibition of ACSL4 significantly reduced FXR-induced ferroptosis in IECs. Transcriptional analysis of intestinal tissues revealed that the deletion of FXR specifically in IECs led to an upregulation of IL-22-related molecules. Considering that IL-22 is predominantly secreted by ILC3s and promotes the production of Reg3b/Reg3g in epithelial cells to maintain gut homeostasis, they focused on ILC3-IL-22 for further study. Flow cytometry analysis showed that IEC-specific FXR knockout mice displayed higher levels of ILC3 frequencies and IL-22 production. Further in vitro co-culture experiments confirmed that oxidized lipids from ferroptotic IECs suppress IL-22 secretion. The administration of intestinal FXR antagonists, ferroptosis inhibitors, or ACSL4 inhibitors significantly enhanced survival rates and decreased intestinal permeability in NEC models. Finally, clinical observations revealed a positive correlation between the levels of oxidized lipids and the severity of NEC in patients.

This study collectively offers novel insights into the pathogenesis of NEC by elucidating the complex interactions among microbiota, intestinal epithelium, and immune cells in the intestines during early infancy. It also underscores the therapeutic potential of targeting intestinal FXR and ferroptosis in the treatment of NEC.
Dr. Jie Zhou from Tianjin Medical University/Tianjin Institute of Immunology, is the corresponding author. Team members Ph.D. candidate Yuxin Zhang, Dr. Yuchao Jing, Dr. Juan He, and Ph.D. candidate Rui Dong, share the co-first authorship.
Article Link: https://doi.org/10.1016/j.immuni.2025.02.003


