Researchers Discover Short IL-18 Generated by Caspase-3 Cleavage in Tumor Cells with Therapeutic Implications for Cancer
Source:Guangxun Meng
2025-03-21
In a study published in Nature Immunology on January 31, which has been selected as the cover article, Prof. Guangxun Meng from the Shanghai Institute of Immunity and Infection (SIII), Chinese Academy of Sciences, and Prof. Chen-ying Liu from Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, unveiled a novel mechanism whereby a short form of IL-18, generated by caspase-3 cleavage in tumor cells, activates NK cells to suppress tumor growth, offering new therapeutic opportunities.
While immunotherapy, including CAR-T cell therapy, has made significant advances, the response rates in treating various solid tumors remain suboptimal, underlining the urgent need for understanding tumor evading mechanisms and innovative treatment strategies. CD8+ T cells and NK cells are the main immune cells conducting immune clearance of tumors. As a key component of the innate immune system, NK cells are increasingly recognized for their potential in cancer immunotherapy due to their rapid response, broad anti-tumor activity, and minimal toxicity. However, tumors employ various mechanisms to evade immune surveillance, such as reducing the expression of key immune modulators including IL-18.
In this study, the researchers focused on IL-18, which is produced as an inactive precursor (pro-IL-18) and requires cleavage by caspase-1 to become the mature, active form. The mature IL-18 is secreted from immune cells such as macrophage, and activates immune cells for tumor control through IL-18 receptor-mediated signal. However, this process is often compromised by a decoy receptor IL-18 binding protein (IL-18BP). In the current work, Prof. Meng’s team discovered that tumor cells can produce a novel short form of IL-18 via caspase-3 cleavage, which is independent of traditional mature IL-18 pathway. Unlike mature IL-18, this short form does not exit the cell but enter the nucleus, where it regulates the phosphorylation of STAT1 and ISG15 secretion, which in turn enhances NK cell anti-tumor function.
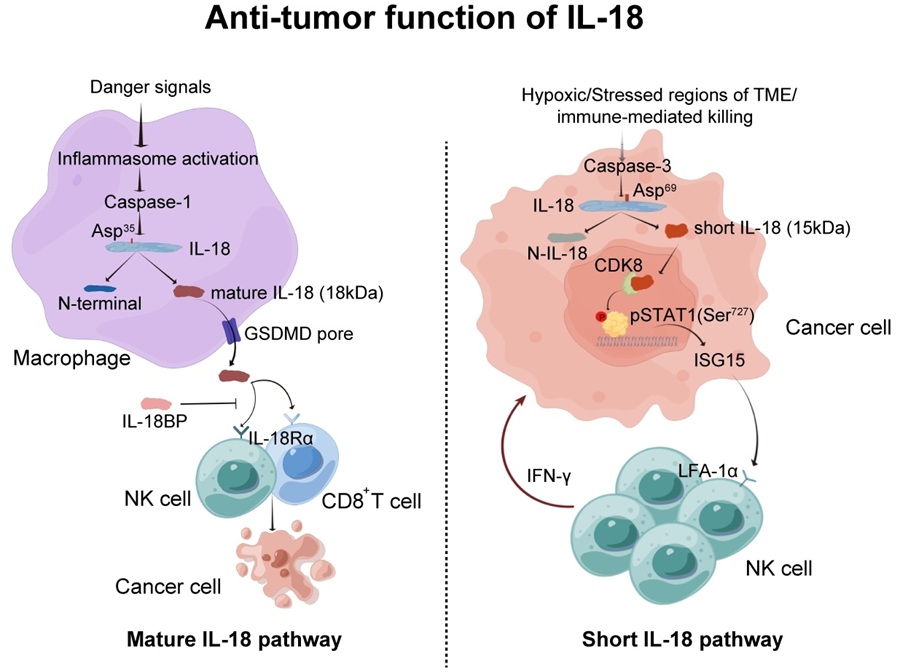
This discovery is particularly relevant in colorectal cancer, wherein the short IL-18 is negatively correlated with tumor progression in clinical samples. Intriguingly, short IL-18 promotes NK cell anti-tumor activity by modulating key signaling pathways, offering a novel and unexpected mechanism through which IL-18 can enhance immune responses in the tumor microenvironment.
“This is a groundbreaking finding that challenges our previous understanding of IL-18,” said Prof. Meng. “By identifying the role of short IL-18 in activating NK cells, we open up new possibilities for developing targeted immunotherapies that may complement existing treatments.”
This study significantly advances our understanding of IL-18’s role in cancer immunotherapy. By inducing short IL-18 expression in tumor cells, the researchers suggest that new therapeutic strategies can be developed to enhance the efficacy of NK cell-based immunotherapies. With further mechanistic studies and clinical validation, these findings may provide a novel therapeutic avenue for cancer patients.
Article Link: https://www.nature.com/articles/s41590-024-02074-7
While immunotherapy, including CAR-T cell therapy, has made significant advances, the response rates in treating various solid tumors remain suboptimal, underlining the urgent need for understanding tumor evading mechanisms and innovative treatment strategies. CD8+ T cells and NK cells are the main immune cells conducting immune clearance of tumors. As a key component of the innate immune system, NK cells are increasingly recognized for their potential in cancer immunotherapy due to their rapid response, broad anti-tumor activity, and minimal toxicity. However, tumors employ various mechanisms to evade immune surveillance, such as reducing the expression of key immune modulators including IL-18.
In this study, the researchers focused on IL-18, which is produced as an inactive precursor (pro-IL-18) and requires cleavage by caspase-1 to become the mature, active form. The mature IL-18 is secreted from immune cells such as macrophage, and activates immune cells for tumor control through IL-18 receptor-mediated signal. However, this process is often compromised by a decoy receptor IL-18 binding protein (IL-18BP). In the current work, Prof. Meng’s team discovered that tumor cells can produce a novel short form of IL-18 via caspase-3 cleavage, which is independent of traditional mature IL-18 pathway. Unlike mature IL-18, this short form does not exit the cell but enter the nucleus, where it regulates the phosphorylation of STAT1 and ISG15 secretion, which in turn enhances NK cell anti-tumor function.

This discovery is particularly relevant in colorectal cancer, wherein the short IL-18 is negatively correlated with tumor progression in clinical samples. Intriguingly, short IL-18 promotes NK cell anti-tumor activity by modulating key signaling pathways, offering a novel and unexpected mechanism through which IL-18 can enhance immune responses in the tumor microenvironment.
“This is a groundbreaking finding that challenges our previous understanding of IL-18,” said Prof. Meng. “By identifying the role of short IL-18 in activating NK cells, we open up new possibilities for developing targeted immunotherapies that may complement existing treatments.”
This study significantly advances our understanding of IL-18’s role in cancer immunotherapy. By inducing short IL-18 expression in tumor cells, the researchers suggest that new therapeutic strategies can be developed to enhance the efficacy of NK cell-based immunotherapies. With further mechanistic studies and clinical validation, these findings may provide a novel therapeutic avenue for cancer patients.
Article Link: https://www.nature.com/articles/s41590-024-02074-7


