Huiyuan Zhang and Hongbo Hu’s group revealed a new pathway for targeting HIF2α in precision asthma therapy
Source:Huiyuan Zhang
2024-12-19
Asthma, a type II hypersensitivity disease primarily caused by the abnormal activation of T helper 2 (Th2) cells, affects over 300 million people worldwide, with the incidence particularly rising in developed countries. While current treatments such as bronchodilators and corticosteroids can effectively alleviate symptoms, they do not cure the disease. Additionally, some severe patients develop drug resistance, leading to recurrent flare-ups. Another concern is the limitation of the efficacy and the tendency for relapse with current cytokine monoclonal antibody therapies. As a result, targeting the pivotal cells responsible for the pathological response in asthma—pathogenic Th2 cells—has become a major focus and challenge in current research.
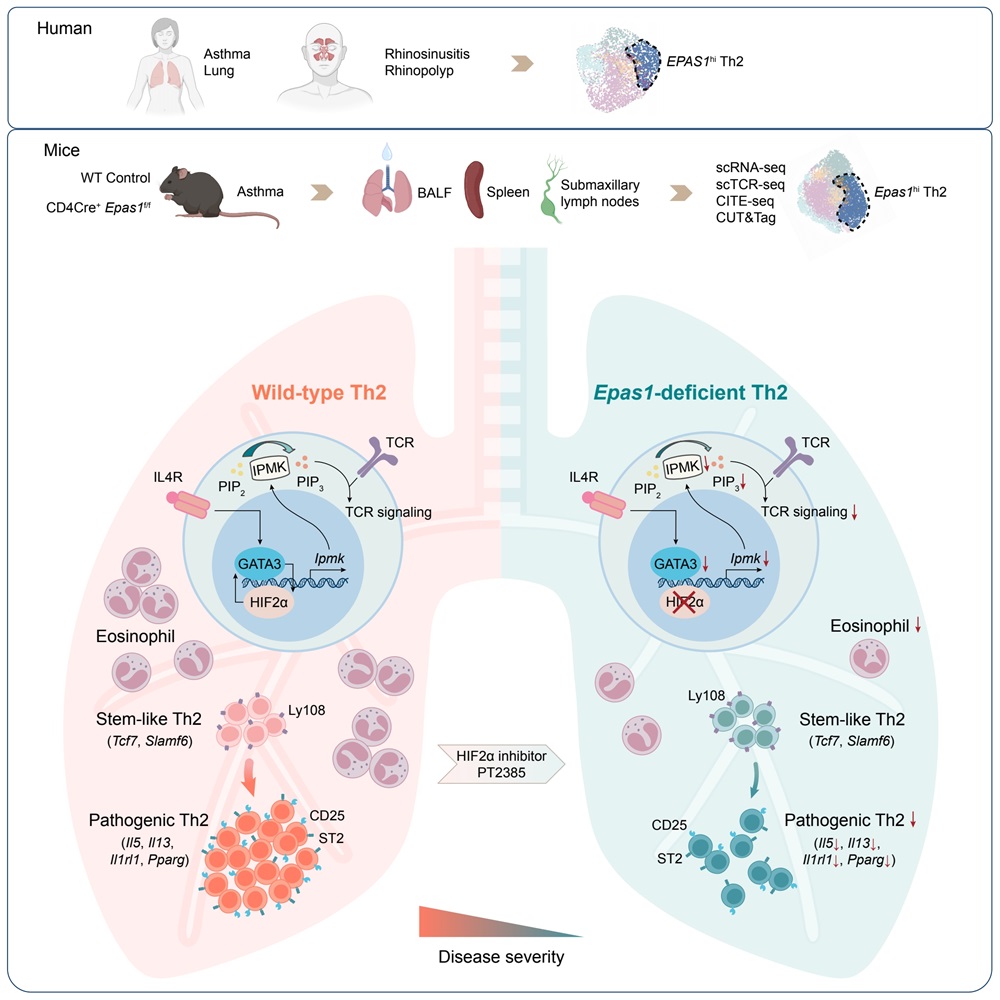
On November 27, 2024, a group led by Professor Huiyuan Zhang and Hongbo Hu from the Center for Immunology and Hematology of Sichuan University, in collaboration with Professor Xindong Liu from the Third Military Medical University, published a research paper titled "Hypoxia-inducible factor 2α promotes pathogenic polarization of stem-like Th2 cells via modulation of phospholipid metabolism" in the prestigious journal Immunity. This study revealed that hypoxia-inducible factor 2α (HIF2α) is specifically highly expressed in pathogenic Th2 cells in both human and mice. By modulating phospholipid metabolism, HIF2α promotes the differentiation of stem-like Th2 cells into pathogenic Th2 cells. This study provides a new molecular target for the precision treatment of asthma and opens up a new therapeutic strategy targeting HIF2α.
The research shows that HIF2α plays a crucial role in the differentiation and function of Th2 cells. Using mice with T cell-specific HIF2α knockout, the researchers found that the absence of HIF2α significantly inhibited Th2 cell differentiation and improved asthma symptoms in mice by reducing pulmonary inflammation. More importantly, HIF2α works in synergy with GATA3, regulating phospholipid metabolism to activate the PI3K-AKT signaling pathway and promote the differentiation of pathogenic Th2 cells. This mechanism provides a new perspective for immunopathological research in asthma and offers theoretical support for precision therapies targeting Th2 cells.
Furthermore, the study demonstrated that the HIF2α inhibitor PT-2385 showed significant effects in asthma treatment. The inhibitor specifically suppressed Th2 cell differentiation and effectively alleviated asthma symptoms in mice OVA-induced asthma models, including reducing the proportion of eosinophils, and lowering the secretion of IL-4, IL-5, and IL-13. This finding provides new insights into HIF2α-targeted therapeutic strategies and brings renewed hope for asthma patients.
This research opens new directions for asthma treatment. HIF2α not only serves as an important regulatory factor in Th2 cell differentiation but also emerges as a potential target for type II immune diseases. The group stated that they will further explore the clinical application of HIF2α inhibitors, aiming to improve asthma treatment outcomes, reduce drug resistance, and enhance clinical efficacy by targeting this key molecule.
The corresponding authors of the paper are Professor Huiyuan Zhang and Hongbo Hu from the Center for Immunology and Hematology of Sichuan University, and Professor Liu Xindong from the Third Military Medical University. The study was supported by the Center for Immunology and Hematology of Sichuan University, West China Hospital, Xuzhou Medical University, and other institutions. Experts from Shanghai Jiao Tong University, Third Military Medical University, and Chongqing Medical University also provided key materials and mice for this research.
Article link: https://www.cell.com/immunity/fulltext/S1074-7613(24)00496-5
On November 27, 2024, a group led by Professor Huiyuan Zhang and Hongbo Hu from the Center for Immunology and Hematology of Sichuan University, in collaboration with Professor Xindong Liu from the Third Military Medical University, published a research paper titled "Hypoxia-inducible factor 2α promotes pathogenic polarization of stem-like Th2 cells via modulation of phospholipid metabolism" in the prestigious journal Immunity. This study revealed that hypoxia-inducible factor 2α (HIF2α) is specifically highly expressed in pathogenic Th2 cells in both human and mice. By modulating phospholipid metabolism, HIF2α promotes the differentiation of stem-like Th2 cells into pathogenic Th2 cells. This study provides a new molecular target for the precision treatment of asthma and opens up a new therapeutic strategy targeting HIF2α.
The research shows that HIF2α plays a crucial role in the differentiation and function of Th2 cells. Using mice with T cell-specific HIF2α knockout, the researchers found that the absence of HIF2α significantly inhibited Th2 cell differentiation and improved asthma symptoms in mice by reducing pulmonary inflammation. More importantly, HIF2α works in synergy with GATA3, regulating phospholipid metabolism to activate the PI3K-AKT signaling pathway and promote the differentiation of pathogenic Th2 cells. This mechanism provides a new perspective for immunopathological research in asthma and offers theoretical support for precision therapies targeting Th2 cells.
Furthermore, the study demonstrated that the HIF2α inhibitor PT-2385 showed significant effects in asthma treatment. The inhibitor specifically suppressed Th2 cell differentiation and effectively alleviated asthma symptoms in mice OVA-induced asthma models, including reducing the proportion of eosinophils, and lowering the secretion of IL-4, IL-5, and IL-13. This finding provides new insights into HIF2α-targeted therapeutic strategies and brings renewed hope for asthma patients.
This research opens new directions for asthma treatment. HIF2α not only serves as an important regulatory factor in Th2 cell differentiation but also emerges as a potential target for type II immune diseases. The group stated that they will further explore the clinical application of HIF2α inhibitors, aiming to improve asthma treatment outcomes, reduce drug resistance, and enhance clinical efficacy by targeting this key molecule.

Model of HIF2α promoting pathogenic Th2 differentiation through the regulation of phospholipid metabolism
The corresponding authors of the paper are Professor Huiyuan Zhang and Hongbo Hu from the Center for Immunology and Hematology of Sichuan University, and Professor Liu Xindong from the Third Military Medical University. The study was supported by the Center for Immunology and Hematology of Sichuan University, West China Hospital, Xuzhou Medical University, and other institutions. Experts from Shanghai Jiao Tong University, Third Military Medical University, and Chongqing Medical University also provided key materials and mice for this research.
Article link: https://www.cell.com/immunity/fulltext/S1074-7613(24)00496-5


