Qing-lei Gao and Ding Ma’ Team Illuminates a Novel Approach to Ovarian Cancer Precision Treatment: Targeting Effector Regulatory T Cells (eTregs)
Source:Qing-lei Gao
2024-11-29
Ovarian cancer, often referred to as the "silent killer," has the highest mortality rate among female malignancies. Despite advancements in molecularly targeted therapies and immunotherapies that have revolutionized treatments for a diverse array of cancers, the 5-year survival rate for advanced ovarian cancer has remained stubbornly around 30%. The current standard of care for newly diagnosed, advanced, unresectable ovarian cancer is platinum-based neoadjuvant chemotherapy followed by surgery. While platinum-based chemotherapy shows high initial response rates, resistance often develops, limiting its ability to improve long-term outcomes. Ovarian cancer is characterized by high genomic instability, particularly in the most aggressive form, high-grade serous ovarian cancer, which frequently harbors BRCA1/2 mutations and results in homologous recombination repair deficiency (HRD). This presents new opportunities for PARP inhibitor therapy based on the principle of synthetic lethality. Although PARP inhibitors have been successfully integrated into clinical practice for various cancers, including ovarian and breast, key questions remain regarding the microenvironmental factors influencing PARP inhibitor response, differences in immune microenvironments between HRD and homologous recombination proficient (HRP), and how treatments modulate these environments remain unanswered.
On September 5, 2024, a collaborative research team led by Academician Ding Ma and Professor Qing-lei Gao from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, in collaboration with Professor Han Liang from the University of Texas MD Anderson Cancer Center, published a groundbreaking study in the journal Cell, titled "Neoadjuvant PARPi or Chemotherapy in Ovarian Cancer Informs Targeting Effector Treg Cells for Homologous-Recombination-Deficient Tumors." This study pioneers the understanding of tumor microenvironmental differences between HRD and HRP ovarian cancer, leveraging multi-omics data from a prospective clinical trial. It elucidates how PARP inhibitors (Niraparib) and chemotherapy reshape the tumor microenvironment, offering new targets and combination therapy strategies for "cold tumors" like ovarian cancer, which typically show poor responses to targeted PD1/PD-L1 therapies.
Professor Qing-lei Gao initiated an innovative, prospective, multicenter Phase II clinical trial (NANT, NCT04507841) to investigate the efficacy and safety of neoadjuvant Niraparib monotherapy for HRD advanced high-grade serous ovarian cancer. This trial provided a robust foundation for analyzing the microenvironmental differences between HRD and HRP ovarian cancers and the effects of PARP inhibitor treatment on these environments.
Capitalizing on the unique design of the NANT trial, the researchers collected paired ovarian cancer tissue and blood samples before and after Niraparib monotherapy and platinum-based chemotherapy. Utilizing a comprehensive approach that included clinical HRD testing, single-cell RNA sequencing, single-cell TCR sequencing, and multiplex immunohistochemistry combined with flow cytometry, they discovered: 1) In HRD-positive ovarian cancer, the immune microenvironment exhibited heightened activation, characterized by an increase in proliferative and IFN-responsive CD4/8+ T lymphocytes; 2) There was a positive correlation between the proportion of IFN-responsive tumor cells and the presence of eTregs, mediated by the IFN-γ-MHC II pathway within tumor cells; 3) The immune status of HRD-positive tumors was negatively modulated by eTregs. Both Niraparib and platinum-based chemotherapy significantly reversed this suppression, leading to a reduction in overall tumor burden as evidenced by decreased CA125 levels.
To explore whether depleting eTregs could sensitize Niraparib treatment, the team constructed various mouse models for verification. CCR8 is one of the specific markers of eTregs. The researchers used the humanized CCR8 monoclonal antibody (ZL-1218) provided by Zai Lab (Shanghai) Co., Ltd. to target and clear eTregs. In both HRD ovarian cancer ID8 and breast cancer E0771 models, the combination of eTreg targeting and Niraparib showed significantly better tumor suppression than Niraparib alone. This effect was related to the downregulation of eTreg proportion. Additionally, CD25 monoclonal antibody-mediated Treg depletion also enhanced the tumor suppressive effects of Niraparib. This study confirms the synergistic antitumor effects of targeted eTreg therapy combined with Niraparib, offering a novel precision immunotherapy approach for ovarian cancer.
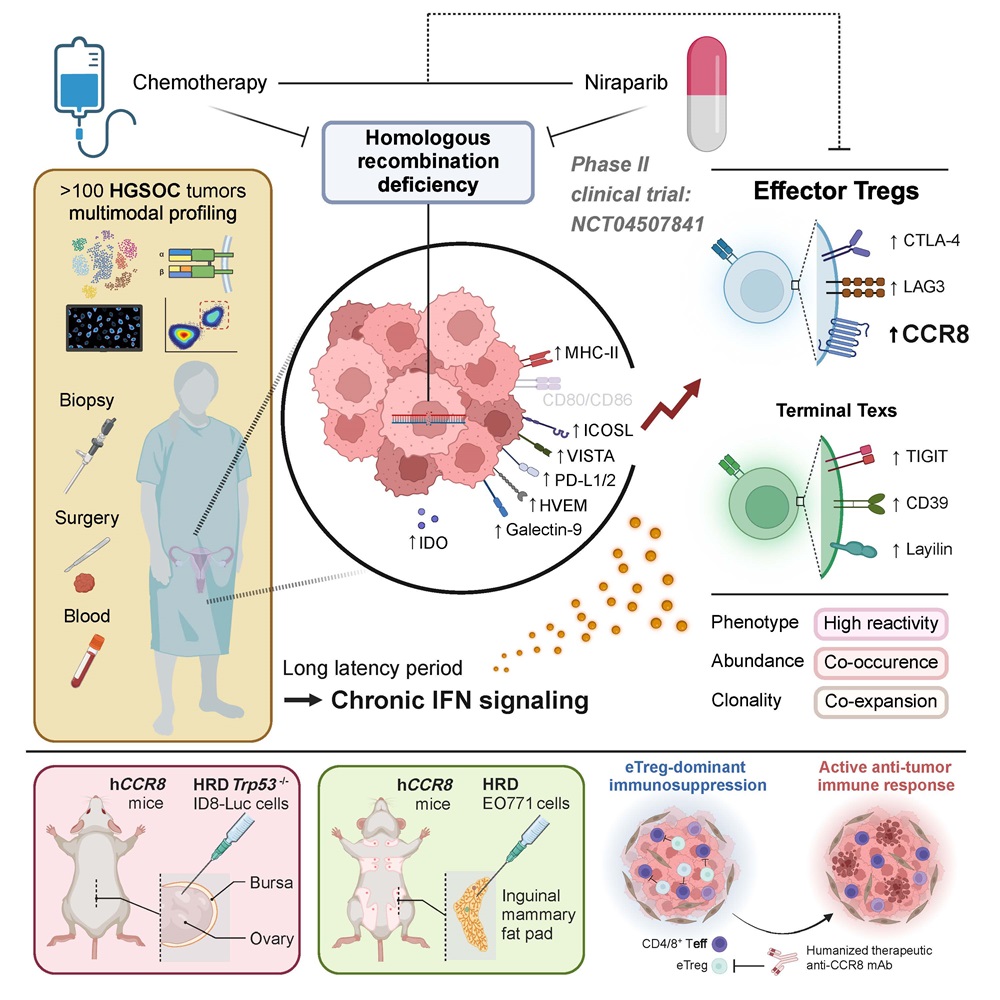
Marking a decade of global clinical use for PARP inhibitors, this study provides the first clinical insight into their impact on reshaping the ovarian cancer microenvironment. It identifies the eTreg as a novel therapeutic target for ovarian cancer immunotherapy, thereby laying the groundwork for future research in precision treatment and microenvironmental studies within the field of ovarian cancer.
The paper's co-first authors include Yikai Luo, Yu Xia, Dan Liu, Xiong Li, Huayi Li, Jiahao Liu, Dongchen Zhou, and Yu Dong. The co-corresponding authors are Professor Qing-lei Gao, Liang Han, Fang Yong, and Ding Ma. This achievement was also supported by Zai Lab (Shanghai) Co., Ltd., Professor Peixiang Lan from Tongji Hospital, Professor Xiangping Yang from Tongji Medical College, Dr. Fanglue Peng from UCSF, and Dr. Zeyu Chen from the Broad Institute.
Link: https://www.sciencedirect.com/science/article/pii/S0092867424006536
On September 5, 2024, a collaborative research team led by Academician Ding Ma and Professor Qing-lei Gao from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, in collaboration with Professor Han Liang from the University of Texas MD Anderson Cancer Center, published a groundbreaking study in the journal Cell, titled "Neoadjuvant PARPi or Chemotherapy in Ovarian Cancer Informs Targeting Effector Treg Cells for Homologous-Recombination-Deficient Tumors." This study pioneers the understanding of tumor microenvironmental differences between HRD and HRP ovarian cancer, leveraging multi-omics data from a prospective clinical trial. It elucidates how PARP inhibitors (Niraparib) and chemotherapy reshape the tumor microenvironment, offering new targets and combination therapy strategies for "cold tumors" like ovarian cancer, which typically show poor responses to targeted PD1/PD-L1 therapies.
Professor Qing-lei Gao initiated an innovative, prospective, multicenter Phase II clinical trial (NANT, NCT04507841) to investigate the efficacy and safety of neoadjuvant Niraparib monotherapy for HRD advanced high-grade serous ovarian cancer. This trial provided a robust foundation for analyzing the microenvironmental differences between HRD and HRP ovarian cancers and the effects of PARP inhibitor treatment on these environments.
Capitalizing on the unique design of the NANT trial, the researchers collected paired ovarian cancer tissue and blood samples before and after Niraparib monotherapy and platinum-based chemotherapy. Utilizing a comprehensive approach that included clinical HRD testing, single-cell RNA sequencing, single-cell TCR sequencing, and multiplex immunohistochemistry combined with flow cytometry, they discovered: 1) In HRD-positive ovarian cancer, the immune microenvironment exhibited heightened activation, characterized by an increase in proliferative and IFN-responsive CD4/8+ T lymphocytes; 2) There was a positive correlation between the proportion of IFN-responsive tumor cells and the presence of eTregs, mediated by the IFN-γ-MHC II pathway within tumor cells; 3) The immune status of HRD-positive tumors was negatively modulated by eTregs. Both Niraparib and platinum-based chemotherapy significantly reversed this suppression, leading to a reduction in overall tumor burden as evidenced by decreased CA125 levels.
To explore whether depleting eTregs could sensitize Niraparib treatment, the team constructed various mouse models for verification. CCR8 is one of the specific markers of eTregs. The researchers used the humanized CCR8 monoclonal antibody (ZL-1218) provided by Zai Lab (Shanghai) Co., Ltd. to target and clear eTregs. In both HRD ovarian cancer ID8 and breast cancer E0771 models, the combination of eTreg targeting and Niraparib showed significantly better tumor suppression than Niraparib alone. This effect was related to the downregulation of eTreg proportion. Additionally, CD25 monoclonal antibody-mediated Treg depletion also enhanced the tumor suppressive effects of Niraparib. This study confirms the synergistic antitumor effects of targeted eTreg therapy combined with Niraparib, offering a novel precision immunotherapy approach for ovarian cancer.

Marking a decade of global clinical use for PARP inhibitors, this study provides the first clinical insight into their impact on reshaping the ovarian cancer microenvironment. It identifies the eTreg as a novel therapeutic target for ovarian cancer immunotherapy, thereby laying the groundwork for future research in precision treatment and microenvironmental studies within the field of ovarian cancer.
The paper's co-first authors include Yikai Luo, Yu Xia, Dan Liu, Xiong Li, Huayi Li, Jiahao Liu, Dongchen Zhou, and Yu Dong. The co-corresponding authors are Professor Qing-lei Gao, Liang Han, Fang Yong, and Ding Ma. This achievement was also supported by Zai Lab (Shanghai) Co., Ltd., Professor Peixiang Lan from Tongji Hospital, Professor Xiangping Yang from Tongji Medical College, Dr. Fanglue Peng from UCSF, and Dr. Zeyu Chen from the Broad Institute.
Link: https://www.sciencedirect.com/science/article/pii/S0092867424006536


