Dr. Qiang Liu's team at Tianjin Medical University General hospital has identified a new immune mechanism underlying post-stroke atheroprogression
Source:Qiang Liu
2024-09-25
Stroke causes a markedly increased risk for recurrent atherothrombotic events in systemic vascular territories. In stroke survivors, the risk of suffering a recurrence is up to 30% by 5 years that is about 9 times the risk of stroke in the general population. The risk is particularly high early after the first stroke, i.e. up to 15% by 1 year that is about 15 times the risk in the general population. These findings indicate that stroke leads to systemic adverse vascular responses, which remains elevated even years after first stroke. Stroke activates the immune system, leading to systemic vascular inflammation. Although stroke-induced monocyte activation and their entry into peripheral arterial plaques have been depicted, little is known about the endothelial-specific alterations in response to stroke and their potential impact on atherosclerotic progression.
Dr. Qiang Liu's group from the Department of Neurology, Tianjin Neurological Institute, Tianjin Medical University General Hospital, published a research article in Immunity on Sep 10, 2024, entitled “Brain ischemia causes systemic Notch1 activity in endothelial cells to drive atherosclerosis”. Using combined approaches of stroke cohort data analysis, single-cell sequencing, two-photon imaging and flow cytometry, they found that brain ischemia induces persistent activation of endothelial Notch1 signaling in the periphery. A sustained increase of circulating Notch1 ligands leads to a senescent, pro-inflammatory endothelium that drives atheroprogression following stroke, which can be therapeutically targeted by blocking antibodies.
Using pooled data from individuals enrolled in the Third China National Stroke Registry (CNSR-III), they found that stroke recurrence and mortality during the first 3 months after stroke onset were mainly caused by atherosclerosis in large artery versus cariogenic embolism or small vessel occlusion. By combining PET-CT and two-photon imaging techniques, they found that brain ischemia induces persistent activation, upregulation of adhesion molecule VCAM1 and increased senescence in peripheral ECs until 4 weeks after stroke onset. This aberrant EC activity resulted from sustained Notch1 signaling, which was triggered by increased circulating Notch1 ligands DLL1 and Jagged1 after stroke in mice and humans. Consequently, this led to increased myeloid cell adhesion and atheroprogression by generating a senescent, pro-inflammatory endothelium (Figure).
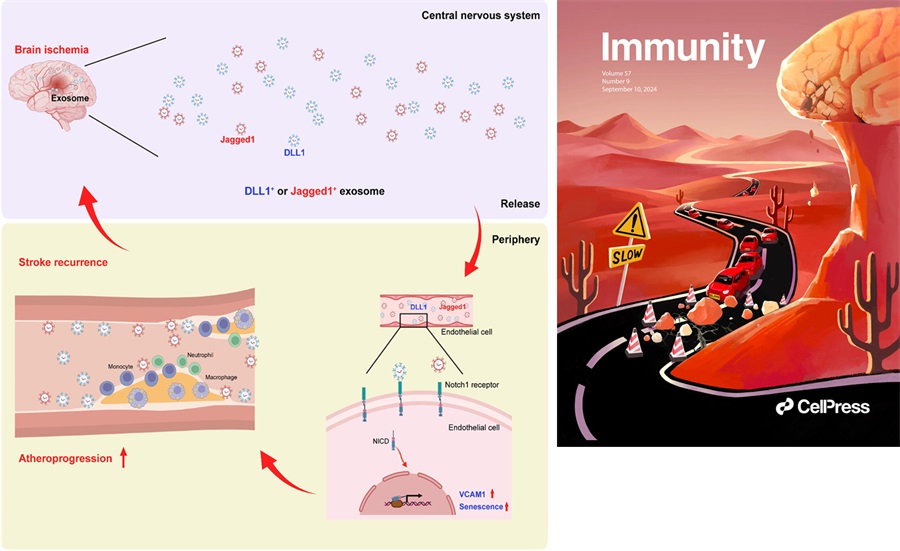
Cover page of the current issue published in Immunity. Brain-derived exosomal Notch1 ligands are illustrated in the form of falling rocks from the top of a mountain that is reminiscent of the human brain. The atheroprogression is depicted as vehicles that cause traffic jam to block the road (i.e. blood traffic congestion) (Right).
Article Link: https://www.cell.com/immunity/abstract/S1074-7613(24)00351-0
Dr. Qiang Liu's group from the Department of Neurology, Tianjin Neurological Institute, Tianjin Medical University General Hospital, published a research article in Immunity on Sep 10, 2024, entitled “Brain ischemia causes systemic Notch1 activity in endothelial cells to drive atherosclerosis”. Using combined approaches of stroke cohort data analysis, single-cell sequencing, two-photon imaging and flow cytometry, they found that brain ischemia induces persistent activation of endothelial Notch1 signaling in the periphery. A sustained increase of circulating Notch1 ligands leads to a senescent, pro-inflammatory endothelium that drives atheroprogression following stroke, which can be therapeutically targeted by blocking antibodies.
Using pooled data from individuals enrolled in the Third China National Stroke Registry (CNSR-III), they found that stroke recurrence and mortality during the first 3 months after stroke onset were mainly caused by atherosclerosis in large artery versus cariogenic embolism or small vessel occlusion. By combining PET-CT and two-photon imaging techniques, they found that brain ischemia induces persistent activation, upregulation of adhesion molecule VCAM1 and increased senescence in peripheral ECs until 4 weeks after stroke onset. This aberrant EC activity resulted from sustained Notch1 signaling, which was triggered by increased circulating Notch1 ligands DLL1 and Jagged1 after stroke in mice and humans. Consequently, this led to increased myeloid cell adhesion and atheroprogression by generating a senescent, pro-inflammatory endothelium (Figure).

Figure. Systemic endothelial activation persists following brain ischemia
Brain ischemia causes a persistent release of brain-derived factors such as Notch 1 ligands that are produced by microglia and carried by exosomes. Microglia-derived exosomal Notch 1 ligands, i.e. DLL1 and Jagged1, bind to Notch 1 receptors on endothelial cells, leading to systemic endothelial activation in the periphery as reflected by upregulation of adhesion molecules on endothelial cells and increased adhesion of myeloid cells. These detrimental alterations accelerate atheroprogression and contribute to increased risk for recurrent vascular events in stroke victims. NICD1: Notch1 intracellular domain, VCAM1: vascular cell adhesion molecule1 (Left).Cover page of the current issue published in Immunity. Brain-derived exosomal Notch1 ligands are illustrated in the form of falling rocks from the top of a mountain that is reminiscent of the human brain. The atheroprogression is depicted as vehicles that cause traffic jam to block the road (i.e. blood traffic congestion) (Right).
Article Link: https://www.cell.com/immunity/abstract/S1074-7613(24)00351-0


