Hai Qi 's team discovered the key factor that safeguards B-cell immune memory
Source:Wen Shao
2024-08-21
When the body is re-infected by the same pathogen, the immune system can elicit a faster and stronger immune response to mitigate or prevent the development of the disease. This immune memory primarily relies on T lymphocytes and B lymphocytes that can recognize the pathogen. The abundance, the lifespan and the recallability of these cells determine the quality of immune memory and the ability of the immune system to resist infection and maintain health. Long-lasting and effective vaccines rely on stimulating the body to produce long-term and robust immune memory. However, it is still unclear how memory lymphocytes can persist and maintain their recallability over a long period.
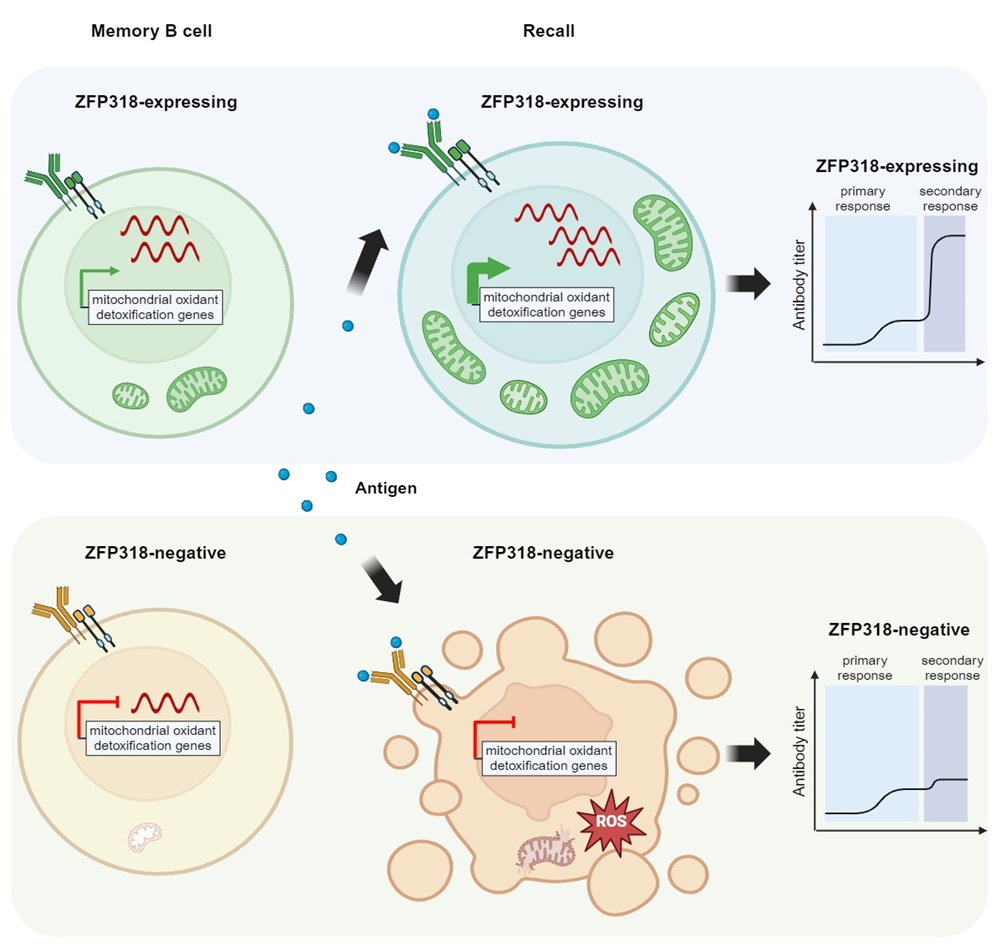
On June 17, 2024, Hai Qi's team from Tsinghua University published a research paper titled "High Recallability of Memory B Cells Requires ZFP318-Dependent Transcriptional Regulation of Mitochondrial Function" in Immunity. The study reported the discovery of ZFP318, the first key regulatory factor specifically maintaining the recallability of memory B cells (MBCs). The authors found that among all MBCs capable of binding to antigens, only a small subset expressing ZFP318 can effectively participate in secondary immune responses. Memory B cells that do not express ZFP318 exhibit mitochondrial dysfunction and rapidly die upon re-encountering antigens due to their inability to resist oxidative stress, thus failing to participate in the secondary response. Animals lacking the ZFP318 gene, while capable of generating a normal primary immune response to vaccines, almost completely lose their immune memory function.
Professor Hai Qi's team has been studying the regulatory mechanisms of B-cell immune memory. In 2017, they identified memory B cell precursors originating from germinal centers. By differential gene expression analysis, they found that the transcriptional regulator ZFP318 was barely expressed in germinal center cells (GCs) but was significantly upregulated in memory B cell precursors and memory B cell populations. To explore the potential function of ZFP318 in memory B cells, the authors constructed a unique ZFP318 mouse reporter/knockout model (Zfp318-loxP-STOP-loxP-DTR-2A-tdTomato, ZSDAT). Immediately downstream of the transcription start site of the Zfp318 gene, they inserted a construct containing a STOP cassette flanked by two loxP sites, which is followed sequentially by the coding sequence for diphtheria toxin receptor (DTR), the coding sequence for the self-cleaving 2A peptide of Thosea asigna virus, and the coding sequence for tdTomato. When crossed with AID-cre mice that specifically express in GC, the offspring could be used for both identifying ZFP318-expressing MBCs derived from germinal centers (ZSDAT+ MBCs) and deleting them. The authors later found that when these ZSDAT+ MBCs were deleted by diphtheria toxin (DT), the mice lost almost all the secondary immune response. These results indicate that although ZSDAT+ MBCs only account for only 10%-20% of all memory B cells, they are the main contributors to secondary immune responses. By comparing wild-type and ZFP318 knockout mice, the authors further discovered that the absence of the ZFP318 gene itself did not affect primary immune responses or serum antibody titers but specifically and significantly impacted the intensity of secondary immune responses and antibody production in mice. Furthermore, the authors constructed B-cell-specific inducible expression mice for ZFP318 (B cell-restricted induction of single-copy knocked-in ZFP318 gene expression, BRISK). They found that forced expression of ZFP318 in MBCs that otherwise hardly express it significantly enhanced their recallabilities, demonstrating that ZFP318 is both sufficient and necessary for the recall of MBC. Through RNA-seq analysis, the authors also found that MBCs lacking ZFP318 expressed relatively lower level of mitochondrial-related genes, particularly those related to reactive oxygen species (ROS) clearance. Accompanying this was abnormal mitochondrial membrane potential and morphology, leading to their increased susceptibility to death upon antigen stimulation. When ZFP318 knockout mice were injected intraperitoneally with N-Acetylcysteine (a ROS scavenger), their secondary immune response capabilities were restored to levels comparable to wild-type mice. These results suggest that the inability to clear ROS is the primary reason for the loss of immune memory in ZFP318-deficient mice. Additionally, the authors found in comparisons of three different vaccines that measuring the abundance of ZFP318-positive memory B cells after primary vaccination could predict the effectiveness of sequential vaccinations, further emphasizing the importance of ZFP318 for B-cell immune memory.
Why is ZFP318 so important yet only expressed in a small subset of MBCs derived from GCs, and what factors control its expression? The authors found that B-cell receptor (BCR) signaling inhibits ZFP318 expression, while only the helper signals from T cells induce ZFP318 expression in GC B cells. This result suggests that memory B cells that have received sufficient help from T cells are the reliable ones that can be recalled upon secondary stimulation, constituting a trustworthy immune memory.
The work of Hai Qi 's team has identified a crucial regulatory molecule essential for B-cell immune memory, revealing the first checkpoint for the recallability of memory B cells. These findings provide new avenues and targets for optimizing vaccine design and achieving long-lasting immune protection in the future. For example, ZFP318 can serve as a biomarker indicating stronger and more durable vaccine efficacy, potentially becoming an alternative endpoint for vaccine evaluation. By enhancing the expression level of ZFP318 in memory B cells, it may be possible to make vaccine protection more robust and long-lasting. The discovery of this novel regulatory mechanism underlying B-cell memory function and its relationship with mitochondria and oxidative stress also seems to suggest similarities between immune memory and our daily life memories: those that are etched in our minds and endure over time are often derived from experiences that have weathered storms and stood the test of time.
Hai Qi, Professor of Tsinghua University is the corresponding author of this research paper. Dr. Yifeng Wang, Associate Researcher at Changping Laboratory, and Dr. Wen Shao, Postdoctoral Fellow at Tsinghua University, are the co-first authors of this paper. The research project received significant contributions from the research groups led by Linqi Zhang from School of Medicine, Li Yu from School of Life Sciences, Guocan Yu from the Department of Chemistry, and Xu Tan from School of Pharmaceutical Sciences at Tsinghua University. The research project was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, Changping Laboratory, Tsinghua-Peking Joint Center for Life Sciences, Beijing Municipal Science and Technology Commission, Beijing Advanced Innovation Center for Structural Biology, and the New Cornerstone Project.
Article Links: https://www.sciencedirect.com/science/article/pii/S1074761324002760
On June 17, 2024, Hai Qi's team from Tsinghua University published a research paper titled "High Recallability of Memory B Cells Requires ZFP318-Dependent Transcriptional Regulation of Mitochondrial Function" in Immunity. The study reported the discovery of ZFP318, the first key regulatory factor specifically maintaining the recallability of memory B cells (MBCs). The authors found that among all MBCs capable of binding to antigens, only a small subset expressing ZFP318 can effectively participate in secondary immune responses. Memory B cells that do not express ZFP318 exhibit mitochondrial dysfunction and rapidly die upon re-encountering antigens due to their inability to resist oxidative stress, thus failing to participate in the secondary response. Animals lacking the ZFP318 gene, while capable of generating a normal primary immune response to vaccines, almost completely lose their immune memory function.
Professor Hai Qi's team has been studying the regulatory mechanisms of B-cell immune memory. In 2017, they identified memory B cell precursors originating from germinal centers. By differential gene expression analysis, they found that the transcriptional regulator ZFP318 was barely expressed in germinal center cells (GCs) but was significantly upregulated in memory B cell precursors and memory B cell populations. To explore the potential function of ZFP318 in memory B cells, the authors constructed a unique ZFP318 mouse reporter/knockout model (Zfp318-loxP-STOP-loxP-DTR-2A-tdTomato, ZSDAT). Immediately downstream of the transcription start site of the Zfp318 gene, they inserted a construct containing a STOP cassette flanked by two loxP sites, which is followed sequentially by the coding sequence for diphtheria toxin receptor (DTR), the coding sequence for the self-cleaving 2A peptide of Thosea asigna virus, and the coding sequence for tdTomato. When crossed with AID-cre mice that specifically express in GC, the offspring could be used for both identifying ZFP318-expressing MBCs derived from germinal centers (ZSDAT+ MBCs) and deleting them. The authors later found that when these ZSDAT+ MBCs were deleted by diphtheria toxin (DT), the mice lost almost all the secondary immune response. These results indicate that although ZSDAT+ MBCs only account for only 10%-20% of all memory B cells, they are the main contributors to secondary immune responses. By comparing wild-type and ZFP318 knockout mice, the authors further discovered that the absence of the ZFP318 gene itself did not affect primary immune responses or serum antibody titers but specifically and significantly impacted the intensity of secondary immune responses and antibody production in mice. Furthermore, the authors constructed B-cell-specific inducible expression mice for ZFP318 (B cell-restricted induction of single-copy knocked-in ZFP318 gene expression, BRISK). They found that forced expression of ZFP318 in MBCs that otherwise hardly express it significantly enhanced their recallabilities, demonstrating that ZFP318 is both sufficient and necessary for the recall of MBC. Through RNA-seq analysis, the authors also found that MBCs lacking ZFP318 expressed relatively lower level of mitochondrial-related genes, particularly those related to reactive oxygen species (ROS) clearance. Accompanying this was abnormal mitochondrial membrane potential and morphology, leading to their increased susceptibility to death upon antigen stimulation. When ZFP318 knockout mice were injected intraperitoneally with N-Acetylcysteine (a ROS scavenger), their secondary immune response capabilities were restored to levels comparable to wild-type mice. These results suggest that the inability to clear ROS is the primary reason for the loss of immune memory in ZFP318-deficient mice. Additionally, the authors found in comparisons of three different vaccines that measuring the abundance of ZFP318-positive memory B cells after primary vaccination could predict the effectiveness of sequential vaccinations, further emphasizing the importance of ZFP318 for B-cell immune memory.
Why is ZFP318 so important yet only expressed in a small subset of MBCs derived from GCs, and what factors control its expression? The authors found that B-cell receptor (BCR) signaling inhibits ZFP318 expression, while only the helper signals from T cells induce ZFP318 expression in GC B cells. This result suggests that memory B cells that have received sufficient help from T cells are the reliable ones that can be recalled upon secondary stimulation, constituting a trustworthy immune memory.
The work of Hai Qi 's team has identified a crucial regulatory molecule essential for B-cell immune memory, revealing the first checkpoint for the recallability of memory B cells. These findings provide new avenues and targets for optimizing vaccine design and achieving long-lasting immune protection in the future. For example, ZFP318 can serve as a biomarker indicating stronger and more durable vaccine efficacy, potentially becoming an alternative endpoint for vaccine evaluation. By enhancing the expression level of ZFP318 in memory B cells, it may be possible to make vaccine protection more robust and long-lasting. The discovery of this novel regulatory mechanism underlying B-cell memory function and its relationship with mitochondria and oxidative stress also seems to suggest similarities between immune memory and our daily life memories: those that are etched in our minds and endure over time are often derived from experiences that have weathered storms and stood the test of time.

ZFP318 safeguards B-cell immune memory
Hai Qi, Professor of Tsinghua University is the corresponding author of this research paper. Dr. Yifeng Wang, Associate Researcher at Changping Laboratory, and Dr. Wen Shao, Postdoctoral Fellow at Tsinghua University, are the co-first authors of this paper. The research project received significant contributions from the research groups led by Linqi Zhang from School of Medicine, Li Yu from School of Life Sciences, Guocan Yu from the Department of Chemistry, and Xu Tan from School of Pharmaceutical Sciences at Tsinghua University. The research project was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, Changping Laboratory, Tsinghua-Peking Joint Center for Life Sciences, Beijing Municipal Science and Technology Commission, Beijing Advanced Innovation Center for Structural Biology, and the New Cornerstone Project.
Article Links: https://www.sciencedirect.com/science/article/pii/S1074761324002760


