Yihua Chen, Feng Shao, and Bian Wu’s teams discovered that the β-D-manno-heptoses are immune agonists across kingdoms
Source:Yihua Chen
2024-08-20
Bacterial small molecule metabolites, such as adenosine-diphosphate-D-glycero-β-D-manno-heptose (ADP-heptose) and their derivatives, act as effective innate immune agonists in mammals. ADP-heptose is synthesized from D-sedoheptulose 7-phosphate (S7P) via a four-step relay catalyzed by NDP-heptose biosynthetic enzymes (HBEs) with isomerase, kinase, phosphatase, and nucleotidyltransferase activities. Knowledge of β-D-manno-heptose biosynthesis is limited to bacteria, and non-bacterial HBEs have not been described yet.
On August 9, 2024, Professor Yihua Chen’s research group from the Institute of Microbiology, Chinese Academy of Sciences (IM, CAS), Professor Feng Shao’s research group from National Institute of Biological Sciences, Beijing, and Professor Bian Wu’s research group from IM, CAS published a paper titled “The β-D-manno-heptoses are immune agonists across kingdoms” in the journal Science. This study found that the β-D-manno-heptoses are cross-kingdom small molecule pathogen-associated molecular patterns that activate the alpha-protein kinase 1 (ALPK1)-dependent innate immune signaling cascade.
In this study, researchers discovered that functional HBEs are distributed widely in bacteria, archaea, eukaryotes, and viruses. A conserved STTR5 motif was identified as a hallmark of heptose nucleotidyltransferases that can synthesize not only ADP-heptose but also cytidine-diphosphate (CDP)- and uridine-diphosphate (UDP)-heptose. Both CDP- and UDP-heptoses are agonists that trigger stronger ALPK1-dependent immune responses than ADP-heptose in human and mouse cells and mice. ADP-heptose was also produced in archaea and verified its innate immune agonist functions.
Evolutionary analysis implied that, after deuterostomes lost the ability to synthesize β-D-manno-heptoses, some vertebrates, including mammals, evolved ALPK1s as specific receptors for immunological recognition of β-D-manno-heptoses produced by members of different kingdoms. Considering the vast number and diverse origins of HBEs, the authors believe that heptose metabolites play many more biological roles than currently known.
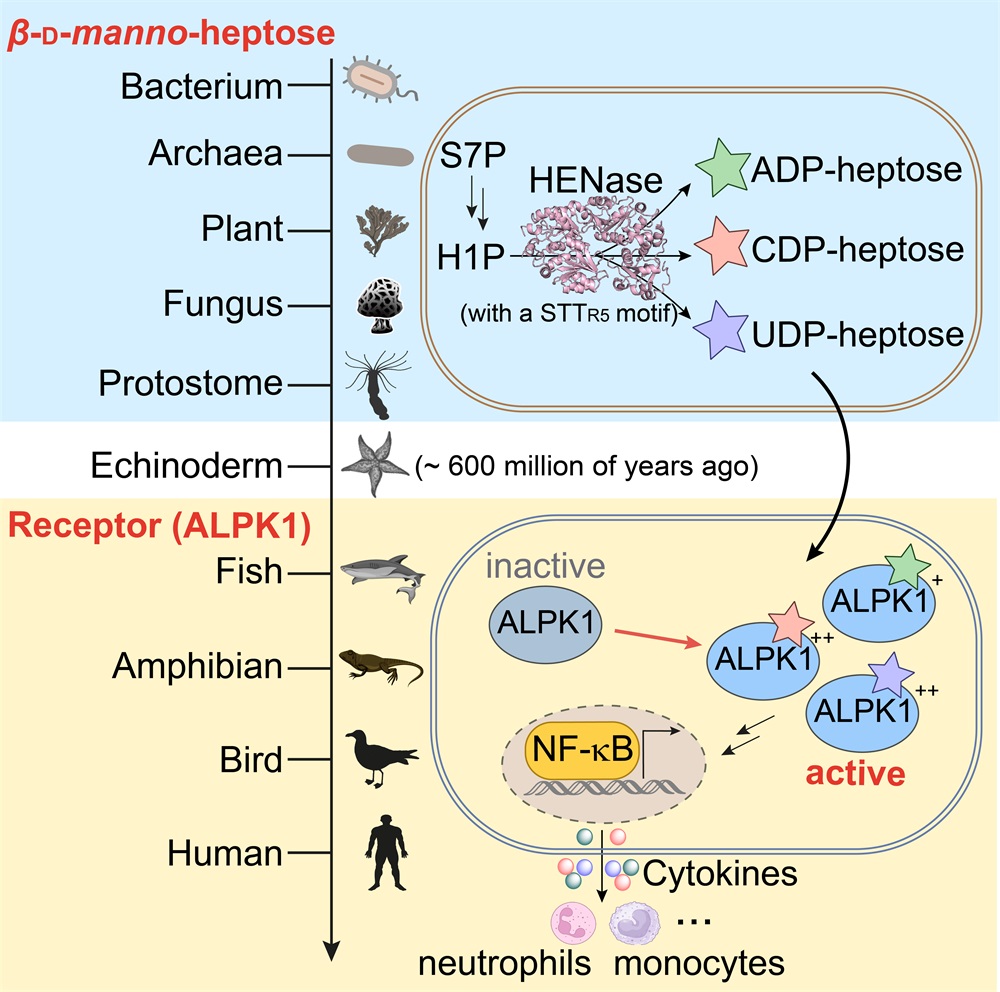
Overall, this study updates the distribution of the β-D-manno-heptose metabolites and sets the stage for future studies regarding the structural diversity of HBE-related heptose metabolites and their biological roles in different kingdoms.
The Institute of Microbiology, Chinese Academy of Sciences is the first affiliated unit for this paper. Professors Yihua Chen, Feng Shao, and Bian Wu are the co-corresponding authors. Postdoctoral researcher Yue Tang, PhD student Xiaoying Tian, associate researchers Min Wang and Yinglu Cui, and Postdoctoral researcher Yang She are the co-first authors of this paper. This work was supported in part by the Ministry of Science and Technology of the People’s Republic of China, the National Natural Science Foundation of China, the Chinese Academy of Sciences, and the Tencent New Cornerstone Investigator Program to Feng Shao.
Article Links: https://www.science.org/doi/10.1126/science.adk7314
On August 9, 2024, Professor Yihua Chen’s research group from the Institute of Microbiology, Chinese Academy of Sciences (IM, CAS), Professor Feng Shao’s research group from National Institute of Biological Sciences, Beijing, and Professor Bian Wu’s research group from IM, CAS published a paper titled “The β-D-manno-heptoses are immune agonists across kingdoms” in the journal Science. This study found that the β-D-manno-heptoses are cross-kingdom small molecule pathogen-associated molecular patterns that activate the alpha-protein kinase 1 (ALPK1)-dependent innate immune signaling cascade.
In this study, researchers discovered that functional HBEs are distributed widely in bacteria, archaea, eukaryotes, and viruses. A conserved STTR5 motif was identified as a hallmark of heptose nucleotidyltransferases that can synthesize not only ADP-heptose but also cytidine-diphosphate (CDP)- and uridine-diphosphate (UDP)-heptose. Both CDP- and UDP-heptoses are agonists that trigger stronger ALPK1-dependent immune responses than ADP-heptose in human and mouse cells and mice. ADP-heptose was also produced in archaea and verified its innate immune agonist functions.
Evolutionary analysis implied that, after deuterostomes lost the ability to synthesize β-D-manno-heptoses, some vertebrates, including mammals, evolved ALPK1s as specific receptors for immunological recognition of β-D-manno-heptoses produced by members of different kingdoms. Considering the vast number and diverse origins of HBEs, the authors believe that heptose metabolites play many more biological roles than currently known.

Overall, this study updates the distribution of the β-D-manno-heptose metabolites and sets the stage for future studies regarding the structural diversity of HBE-related heptose metabolites and their biological roles in different kingdoms.
The Institute of Microbiology, Chinese Academy of Sciences is the first affiliated unit for this paper. Professors Yihua Chen, Feng Shao, and Bian Wu are the co-corresponding authors. Postdoctoral researcher Yue Tang, PhD student Xiaoying Tian, associate researchers Min Wang and Yinglu Cui, and Postdoctoral researcher Yang She are the co-first authors of this paper. This work was supported in part by the Ministry of Science and Technology of the People’s Republic of China, the National Natural Science Foundation of China, the Chinese Academy of Sciences, and the Tencent New Cornerstone Investigator Program to Feng Shao.
Article Links: https://www.science.org/doi/10.1126/science.adk7314


