Hai Qi's team discovered the regulatory mechanism governing memory B cell differentiation -- the epigenetically recorded stimulation history of B cells
Source:Wen Shao
2024-08-19

B cell differentiation: a kid running towards the finish line
When the body is infected by the same pathogen, some memory B cells in the body can rapidly differentiate into antibody-producing plasma cells to resist the invasion of the pathogen, while others form secondary germinal centers (GCs) for further somatic hypermutation and affinity maturation. MBC re-participation in the GC reaction is thought to be important for generating broadly neutralizing antibodies against highly mutating viruses such as influenza and HIV. Distinct isotypes and MBC surface phenotypes are found to associate with different choices of developing into secondary GCs or PCs However, how MBCs are intrinsically programmed by transcriptional and epigenetic mechanisms to produce secondary GCs or PCs is not yet understood.
On July 5, 2024, Hai Qi's research team from Tsinghua University published a research paper titled "Epigenetic Recording of Stimulation History Reveals BLIMP1–BACH2 Balance in Determining Memory B Cell Fate upon Recall Challenge" in Nature Immunology. This paper discovered the molecular mechanism that regulates the re-differentiation fate of memory B cells. The authors found that the cumulative stimulation history that B cells experience is epigenetically recorded in an IRF4-dependent manner, determines the relative strength between BLIMP1 and BACH2 in B cells, including memory B cells, and in turn dictates the probability of individual memory B cells to develop into GCs or PCs upon re-stimulation.
BACH2 and BLIMP1 are two antagonistic transcription factors that are required for GC and PC formation, respectively, and they are components of the double-negative feedback gene regulatory network that orchestrates B cell fate determination. To examine varying BLIMP1 and BACH2 expression at the single-cell level, Hai Qi's team bred the BACH2tdRFP/+ knock-in reporter strain and the BAC-transgenic BLIMP1-EYFP reporter strain to obtain a strain (abbreviated as BARBE, BACH2-RFP-BLIMP1-EYFP) carrying both reporters, which allowed for simultaneous monitoring of BACH2 and BLIMP1 expression. By immunizing BARBE mice, the authors found that while BACH2 expression gradually decreases across post-activation stages of B cells, from the naïve state to GC, MBC, and the terminal PC state. BLIMP1 gradually increases as B cells traverse the same course. GCs or MBCs from later time points of a primary response exhibit increased BLIMP1 and decreased BACH2 expression. Additionally, the authors found MBCs that are predisposed for development of secondary GCs (e.g., IgM+ or CD80-PDL2-) exhibit higher BACH2 but lower BLIMP1 than those predisposed for PC formation (such as IgG+ or CD80+PDL2+ MBCs). These results indicate that the relative levels of BLIMP1 and BACH2 (the ratio of BLIMP1 to BACH2, abbreviated as BLIBA) are closely associated with the differentiation fate of B cells.
To test whether the relative strength of BLIMP1 and BACH2 controls the decision of MBCs in producing PCs or GCs during the recall, the authors constructed a B cell-specific inducible expression mouse model (BRISK, B cell-restricted induction of single-copy knocked-in BACH2/BLIMP1 gene expression). They found BACH2 induction markedly increases GC formation by otherwise PC-predisposed MBCs (IgG+ or CD80+PDL2+), whereas BLIMP1 induction drives PC formation by otherwise GC-prone MBCs (IgM+ or CD80-PDL2-). These results demonstrate that the fate determination of memory B cells is not causally linked to their subtypes or surface molecules but is intrinsically determined by the relative expression levels of these two transcription factors.
The BLIMP1-BACH2 balance progressively changes in favor of BLIMP1 as B cells traverse intermediate states of differentiation toward PCs and through the course of the response. While the progressive BACH2 downregulation in MBCs was apparent, BLIMP1’s upregulation exhibits a switch-like behavior. In biological systems, bidirectional negative feedback gene regulatory networks often lead to such jumps in phenotypic changes. Although BLIMP1 protein is expressed only at a very low level or not at all in naïve B cells, bulk MBCs or GCs, the potential to strongly upregulate BLIMP1 to surpass the switching threshold could be progressively increased as any given B cell clones continuously undergo stimulation during an immune response. To explore such mechanisms, the authors conducted transposase-accessible chromatin with sequencing (ATAC-seq) assays to probe chromatin accessibility features that can distinguish MBCs with the lowest and highest BLIBA. Analysis revealed a higher similarity in the global epigenetic landscape between PCs and EYFP+ MBCs as opposed to RFPhigh MBCs. EYFP+ MBCs displayed more chromatin accessibilities at PC-specific peaks, especially at those IRF4-targeting sites. IRF4 is a critical regulator of B cell differentiation that directly promotes BLIMP1 expression while directly or indirectly inhibiting BACH2 expression. They observed progressively increased chromatin accessibilities at genomic loci that are specifically opened in PCs, particularly those that contain ISRE motifs and are regulated by IRF4, including the Prdm1 locus.
IRF4 is an immediate early gene downstream of BCR signaling. Activation of B cells with anti-Ig or anti-CD40 antibody or CpG led to rapid upregulation of IRF4 mRNA expression in a dose-dependent manner. Many cytokines, including Il-2, Il-4 and Il-21, and additional innate stimuli that are known to promote B cell differentiation toward PCs, also increased IRF4 expression. High-dose of anti-Ig stimulation leads to a significant increase in the chromatin openness of IRF4-targeted PC-specific loci. These results suggest that the history of immune stimulation experienced by B cells is constantly recorded in an IRF4-dependent epigenetic manner. The authors then immunized S1PR2-CreERT2;Rosa26-Ai14 mice and pulse-labeled GC and GC-derived cells by tamoxifen administration from day 6 to 8 and analyzed chromatin features of tdTomato+ GC light-zone (LZ) B cells on day 10 and day 21 post-immunization. TdTomato+ cells at these two time points are phenotypically identical GC LZ cells and have derived from GC cells labeled between day 6 and 8. Day-21 cells should have on average experienced a longer history of stimulation by antigen and T cell help. The results showed that chromatin from cells on day 21 exhibited higher openness at IRF4-targeted loci containing ISRE motifs. This finding indicates that even GC B cells that barely express BLIMP1 still record the stimulation history through epigenetic mechanisms that determine cell fate. These results also explain why GC B cells in the later stages of the immune response produce more plasma cells than those in the early stages.
This work has uncovered the molecular mechanism governing the fate choice of memory B cells, and proposed for the first time that it is the epigenetic recording of the stimulation history of each B cell that determines probability of individual memory B cell to develop into GCs or PCs upon re-stimulation. This epigenetic recording process occurs during the entire lifespan of each B cell and its descendants. This achievement suggests that for vaccines aimed at inducing immunity against highly mutable viruses such as HIV, delaying the accumulation of IRF4-dependent epigenetic imprints may promote the re-engagement of vaccine-induced memory B cells in GC reactions, thereby enhancing the potential for generating broadly neutralizing antibodies. The relationship between B-cell fate determination and the underlying epigenetic regulatory accumulation also seems to hint at the similarities between cell differentiation fate and our lives: Behind every apparent effortless success lies years of accumulated effort and perseverance, akin to water dripping constantly on a stone; those seemingly insignificant trials and tribulations are constantly shaping us.
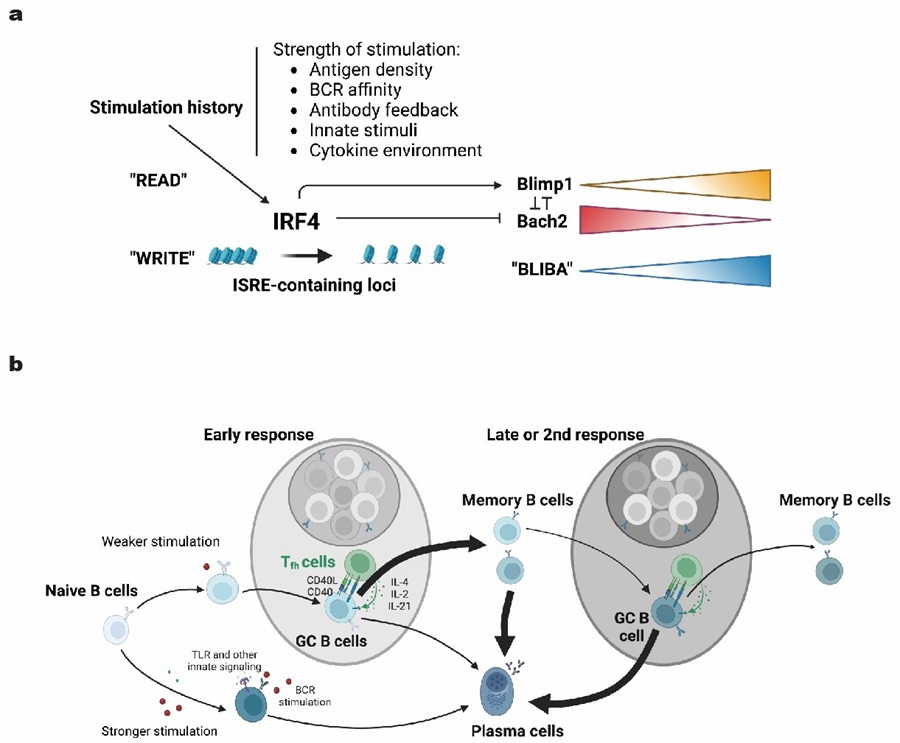
The IRF4 imprinting model and implications for the B cell response
Professor Hai Qi from Tsinghua University, is the corresponding author of this paper. Dr. Wen Shao, a postdoctoral fellow at Tsinghua University, and Dr. Yifeng Wang, an associate researcher at Changping Laboratory, are the co-first authors of this paper. The research project is supported by the National Key Research and Development Program of Chin, the National Natural Science Foundation of China, Changping Laboratory, Tsinghua-Peking University Joint Center for Life Sciences, Beijing Municipal Science and Technology Commission, Beijing Advanced Innovation Center for Structural Biology, and the New Stone Project.
Article Links: https://www.nature.com/articles/s41590-024-01900-2


