Prof. Shu Zhu and Prof. Wen Pan’s group reveal the mechanism and treatment of microbiota-mediated immune escape in colorectal cancer
Source:Shu Zhu
2024-05-14
On March 12, 2024, Professor Shu Zhu and Professor Wen Pan from the University of Science and Technology of China published a research article entitled "Bile acids modified by the intestinal microbiota promote colorectal cancer growth by suppressing CD8+ T cell effector functions" in Immunity. The research findings revealed the microbe C. scindens-mediated tumor immune escape mechanism in colorectal cancer. Additionally, they developed a phage-based therapy. These have potential implications for the prevention of specific colorectal cancer risk groups, immunotherapy for specific colorectal cancer patients, and control of postoperative recurrence.
Colorectal cancer is one of the malignant tumors of the digestive system. Globally, it ranks third in terms of new cases among men and second among women, and fourth in terms of deaths among men and third among women. In China, the incidence of colorectal cancer is increasing year by year. It ranks second in terms of new cases and fourth in terms of deaths among all malignant tumors.
Colorectal cancer's high incidence and low response rates to immunotherapy underscore the urgent need to identify environmental impediments in order to find more precise and effective treatment modalities. It is well known that the colon harbors a large number of intestinal microbiota. Over the past few decades, researchers have discovered that Salmonella typhi and Fusobacterium nucleatum can both activate the Wnt/β-catenin pathway and become risk factors for colorectal cancer. However, this is just the tip of the iceberg in terms of the impact of the 1013 bacteria present in the gut on the host. In a paper published in Nature Cell Biology in January of this year, the Prof. Shu Zhu collaborated with Prof. Bo Chu's team from Shandong University and found that the Peptostreptococcus anaerobius is enriched in the intestines of colorectal cancer patients. P. anaerobius secretes the tryptophan metabolite IDA, which is a natural ligand of AHR. By activating the intestinal epithelial cell ALDH1A3-FSP1-CoQ10 axis to resist ferroptosis, it has adverse effects on tumor prognosis. In fact, the intestine not only contains a large number of epithelial cells but also harbors more than half of the body's immune cells and over 70% of T cells, making it the largest mucosal immune organ in the body.
CD8+ T cells are the core immune cells that resist tumors. In the early stages of tumor development, initial CD8+ T cells receive antigen and co-stimulatory signals, initiating TCR signal transduction, increasing intracellular Ca2+ concentration, and initiating the transcription of effector molecules such as IFN-γ and TNF by nuclear translocation of transcription factor NFAT, thereby activating CD8+ T cells. Activated CD8+ T cells help the body clear tumor cells by secreting cytokines and releasing Granzyme B through pathways. After colorectal cancer occurs, the function of infiltrating CD8+ T cells in the tumor microenvironment is abnormal. The quantity and gene expression pattern of infiltrating CD8+ T cells are important prognostic indicators of colorectal cancer. High proportions of CD8+ T cell infiltration and high expression of Granzyme B signify a good prognosis. However, whether the dysfunction of CD8+ T cells in the colorectal cancer tumor microenvironment is related to altered intestinal bacteria/metabolites, and which bacteria/metabolites affect the anti-tumor function of CD8+ T cells, are still largely unknown.
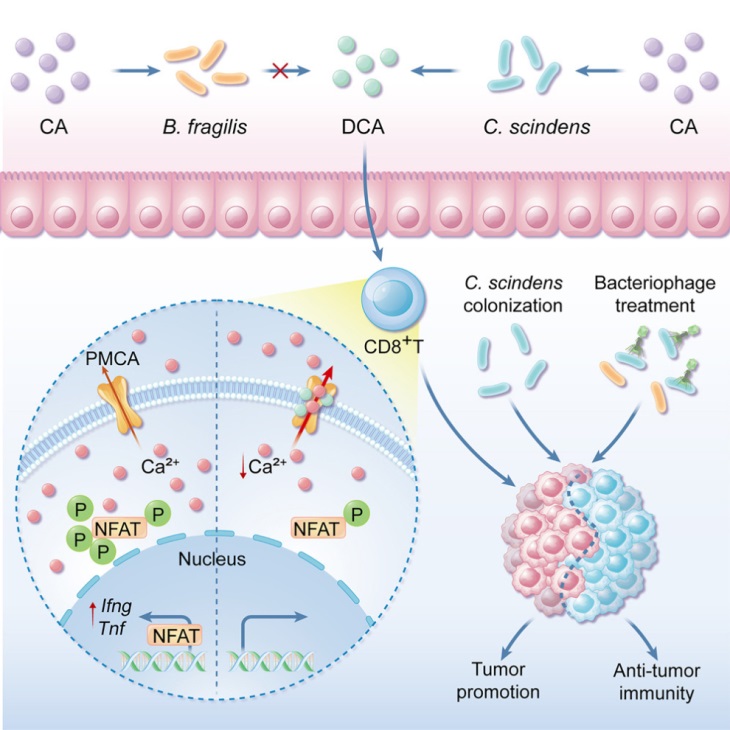
To identify bacteria/metabolites that regulate the anti-tumor function of CD8+ T cells, the research team constructed a library of small molecule metabolites derived from intestinal microbiota. Through in vitro screening and in vivo validation, they discovered that Clostridium scindens produces the secondary bile acid deoxycholic acid (DCA) via the bai operon, which effectively inhibits the effector function of CD8+ T cells and promotes the growth of colorectal cancer. Further studies revealed that DCA may bind to the cell membrane calcium transporter PMCA, enhancing its activity, thereby promoting its mediated Ca2+ efflux, resulting in decreased cytoplasmic Ca2+ concentration, inhibition of NFAT2 activation, and consequently weakening the effector function of CD8+ T cells. Blocking the bacterial DCA biosynthesis pathway effectively inhibits the bacterial suppression of CD8+ T cell effector function, thereby blocking the effect of DCA on mouse tumor growth. Specifically targeting phages to lyse C. scindens effectively inhibits the immunosuppression and tumor-promoting effects of C. scindens. This study reveals a new pathway of interaction between the intestinal microbiota environment and the tumor immune microenvironment, with potential significance for preventing the suppression of immune cells by specific microbes, thereby preventing specific groups at risk of colorectal cancer, immunotherapy for specific colorectal cancer patients, and control of postoperative recurrence.
Prof. Shu Zhu and Prof. Wen Pan from the University of Science and Technology of China are the corresponding authors of the article. Postdoctoral fellow Jingjing Cong from the Shu Zhu group (now a professor at Anhui Medical University) and doctoral student Pianpian Liu from the Wen Pan group, and doctoral student Zili Han from the Shu Zhu group are the co-first authors of this work. This work also received substantial assistance from several collaborators: Prof. Linfeng Sun and Prof. Chunlei Cang from the University of Science and Technology of China provided support for DCA regulation of calcium transporter activity and calcium ion imaging; Prof. Lei Dai from the Shenzhen Advanced Institute, Prof. Chunjun Guo from Cornell University, and Prof. Xinyang Song from the Shanghai Institute of Biochemistry provided support for microbial editing and phage screening; Prof. Dennis Kasper from Harvard University and Prof. Xinyang Song from the Shanghai Institute of Biochemistry provided support for excluding the traditional receptor-mediated effects of DCA on CTLs; Director Yu Bo and Dr. Shuling Wang from the Digestive Department of Shanghai Changzheng Hospital provided support for CRC patient correlation analysis. In addition, Prof. Rongbin Zhou, Prof. Congzhao Zhou from the University of Science and Technology of China, and Prof. Lixin Ye from the Army Medical University provided substantial experimental support and guidance.
Article link: https://doi.org/10.1016/j.immuni.2024.02.014
Colorectal cancer is one of the malignant tumors of the digestive system. Globally, it ranks third in terms of new cases among men and second among women, and fourth in terms of deaths among men and third among women. In China, the incidence of colorectal cancer is increasing year by year. It ranks second in terms of new cases and fourth in terms of deaths among all malignant tumors.
Colorectal cancer's high incidence and low response rates to immunotherapy underscore the urgent need to identify environmental impediments in order to find more precise and effective treatment modalities. It is well known that the colon harbors a large number of intestinal microbiota. Over the past few decades, researchers have discovered that Salmonella typhi and Fusobacterium nucleatum can both activate the Wnt/β-catenin pathway and become risk factors for colorectal cancer. However, this is just the tip of the iceberg in terms of the impact of the 1013 bacteria present in the gut on the host. In a paper published in Nature Cell Biology in January of this year, the Prof. Shu Zhu collaborated with Prof. Bo Chu's team from Shandong University and found that the Peptostreptococcus anaerobius is enriched in the intestines of colorectal cancer patients. P. anaerobius secretes the tryptophan metabolite IDA, which is a natural ligand of AHR. By activating the intestinal epithelial cell ALDH1A3-FSP1-CoQ10 axis to resist ferroptosis, it has adverse effects on tumor prognosis. In fact, the intestine not only contains a large number of epithelial cells but also harbors more than half of the body's immune cells and over 70% of T cells, making it the largest mucosal immune organ in the body.
CD8+ T cells are the core immune cells that resist tumors. In the early stages of tumor development, initial CD8+ T cells receive antigen and co-stimulatory signals, initiating TCR signal transduction, increasing intracellular Ca2+ concentration, and initiating the transcription of effector molecules such as IFN-γ and TNF by nuclear translocation of transcription factor NFAT, thereby activating CD8+ T cells. Activated CD8+ T cells help the body clear tumor cells by secreting cytokines and releasing Granzyme B through pathways. After colorectal cancer occurs, the function of infiltrating CD8+ T cells in the tumor microenvironment is abnormal. The quantity and gene expression pattern of infiltrating CD8+ T cells are important prognostic indicators of colorectal cancer. High proportions of CD8+ T cell infiltration and high expression of Granzyme B signify a good prognosis. However, whether the dysfunction of CD8+ T cells in the colorectal cancer tumor microenvironment is related to altered intestinal bacteria/metabolites, and which bacteria/metabolites affect the anti-tumor function of CD8+ T cells, are still largely unknown.

To identify bacteria/metabolites that regulate the anti-tumor function of CD8+ T cells, the research team constructed a library of small molecule metabolites derived from intestinal microbiota. Through in vitro screening and in vivo validation, they discovered that Clostridium scindens produces the secondary bile acid deoxycholic acid (DCA) via the bai operon, which effectively inhibits the effector function of CD8+ T cells and promotes the growth of colorectal cancer. Further studies revealed that DCA may bind to the cell membrane calcium transporter PMCA, enhancing its activity, thereby promoting its mediated Ca2+ efflux, resulting in decreased cytoplasmic Ca2+ concentration, inhibition of NFAT2 activation, and consequently weakening the effector function of CD8+ T cells. Blocking the bacterial DCA biosynthesis pathway effectively inhibits the bacterial suppression of CD8+ T cell effector function, thereby blocking the effect of DCA on mouse tumor growth. Specifically targeting phages to lyse C. scindens effectively inhibits the immunosuppression and tumor-promoting effects of C. scindens. This study reveals a new pathway of interaction between the intestinal microbiota environment and the tumor immune microenvironment, with potential significance for preventing the suppression of immune cells by specific microbes, thereby preventing specific groups at risk of colorectal cancer, immunotherapy for specific colorectal cancer patients, and control of postoperative recurrence.
Prof. Shu Zhu and Prof. Wen Pan from the University of Science and Technology of China are the corresponding authors of the article. Postdoctoral fellow Jingjing Cong from the Shu Zhu group (now a professor at Anhui Medical University) and doctoral student Pianpian Liu from the Wen Pan group, and doctoral student Zili Han from the Shu Zhu group are the co-first authors of this work. This work also received substantial assistance from several collaborators: Prof. Linfeng Sun and Prof. Chunlei Cang from the University of Science and Technology of China provided support for DCA regulation of calcium transporter activity and calcium ion imaging; Prof. Lei Dai from the Shenzhen Advanced Institute, Prof. Chunjun Guo from Cornell University, and Prof. Xinyang Song from the Shanghai Institute of Biochemistry provided support for microbial editing and phage screening; Prof. Dennis Kasper from Harvard University and Prof. Xinyang Song from the Shanghai Institute of Biochemistry provided support for excluding the traditional receptor-mediated effects of DCA on CTLs; Director Yu Bo and Dr. Shuling Wang from the Digestive Department of Shanghai Changzheng Hospital provided support for CRC patient correlation analysis. In addition, Prof. Rongbin Zhou, Prof. Congzhao Zhou from the University of Science and Technology of China, and Prof. Lixin Ye from the Army Medical University provided substantial experimental support and guidance.
Article link: https://doi.org/10.1016/j.immuni.2024.02.014


