Liangjing Wang and Shujie Chen group reveals a new mechanism by which intestinal bacteria cooperate to facilitate tumor immunotherapy
Source:Liangjing Wang
2024-05-06
Gut microbiota is an important environmental factor that influences the efficacy of immune checkpoint blockade (ICB). The composition of the gut microbiota varies in different tumor patients, and fecal microbiota transplantation (FMT) has been shown to improve ICB responsiveness. Although a growing number of studies have shown that specific bacterial strains can influence ICB efficacy, the specific components and intrinsic mechanisms remain to be elucidated. T cells that infiltrate the tumor microenvironment play a key role in ICB therapy. Recent studies have shown that progenitor exhausted CD8+ T cells (Tpex) with high expression of the transcription factor T-cell factor 1 (TCF-1, encoded by TCF7) and immunocyte stemness properties are a key subset responding to immunotherapy with anti-PD-1 antibodies. Tcf7 knockout mice are associated with an imbalance in gut microbiota, but the ability of specific strains and their metabolites to modulate the immunocyte stemness to increase sensitization to immunotherapy has not been reported.
On March 2024, Professor Liangjing Wang's team (Second Affiliated Hospital of Zhejiang University School of Medicine) and Professor Shujie Chen's team (Sir Run Run Shaw Hospital, Zhejiang University) jointly published a research article in Cell entitled ‘Microbial metabolite enhances immunotherapy efficacy by modulating T-cell stemness in pan-cancer’. The researchers report that the abundance of commensal Lactobacillus johnsonii is positively correlated with the responsiveness of ICB. Supplementation with Lactobacillus johnsonii or tryptophan-derived metabolite indole-3-propionic acid (IPA) enhances the efficacy of CD8+ T cells-mediated αPD-1 immunotherapy. Mechanistically, Lactobacillus johnsonii collaborates with Clostridium sporogenes to produce IPA. IPA modulates the stemness program of CD8+ T cells and facilitates the generation of Tpex cells by increasing H3K27 acetylation at the super-enhancer region of Tcf7. IPA improves ICB responsiveness at the pan-cancer level including melanoma, breast cancer and colorectal cancer. Collectively, the findings identify a microbial metabolite-immune regulatory pathway and suggest a potential microbial-based adjuvant approach to improve the responsiveness of immunotherapy.
In this study, the authors firstly found that there were ‘responsive’ and ‘poorly responsive’ mice to immunotherapy similar to clinical practice, and verified that gut microbiota can influence the efficacy of tumor immunotherapy based on FMT; through sequencing and screening of gut microbiota, the researchers independently isolated a strain of Lactobacillus johnsonii (L. j), which increased the efficacy of CD8+ T-cell infiltration and sensitization of anti-PD1 therapy; the researchers then explored the efficacy components of L. j, compared with heat-inactivated and ultrasonically fragmented bacteria, the culture supernatant of L. j had similar effects as live L. j, suggesting that bacterial metabolites may play a key role; by using LC-MS/MS, the researchers targeted the tryptophan metabolic pathway and the key ingredient: indole-3-propionic acid (IPA).
The researchers used Rag1-/- mice, anti-CD8 neutralizing antibody, and adoptive cell transfer therapy to validate the sensitizing immunotherapeutic effects of L. j and its derived IPA dependent on CD8+ T cells, and further sorted CD8+ T cells infiltrated in tumors of mice for single-cell RNA sequencing (scRNA-seq), single-cell TCR sequencing (scTCR-seq) and single-cell Assay for Targeting Accessible-Chromatin with high-throughout sequencing (scATAC-seq); gavage IPA significantly increased the proportion of ‘stem cell-like’ Tpex cells and effector CD8+ T cells, Tcf7-/- mice verified that IPA requires Tpex cells for sensitizing immunotherapeutic effects; combined with single-cell transcriptomic pathway enrichment and scATAC-seq, the authors found that IPA regulates chromatin opening in Tpex cells through histone acetylation modifications; western blot, ChIP, CUT & RUN, and CUT & Tag sequencing further revealed that IPA increases H3K27 acetylation in the Tcf7 super-enhancer region.
The authors then explored how L. j produces IPA; interestingly, IPA was not detected in L. j culture supernatants in vitro, but instead the upstream metabolite of IPA, indole-3-lactic acid (ILA) was detected; based on the antibiotic cocktail model and intraperitoneal injection model, the authors hypothesized that ILA produced by L. j is further metabolized to IPA by other gut microbiota in vivo and verified this in germ-free mice model; the authors also predicted that ldhA, was the key enzyme for ILA production by L. j and transformed a ldhA expressional vector into E. coli. E. coli overexpressing ldhA increased IPA production and sensitized immunotherapeutic effects. Finally, the investigators verified the regulatory effects of IPA on Tpex cells and immunotherapy in a variety of mouse tumor models (both breast cancer and melanoma transplantable tumor model, mammary fat pad orthotopic implantation model, MMTV-PyMT spontaneous breast cancer model and cecum orthotopic implantation model) and in organoids derived from colorectal cancer patients.
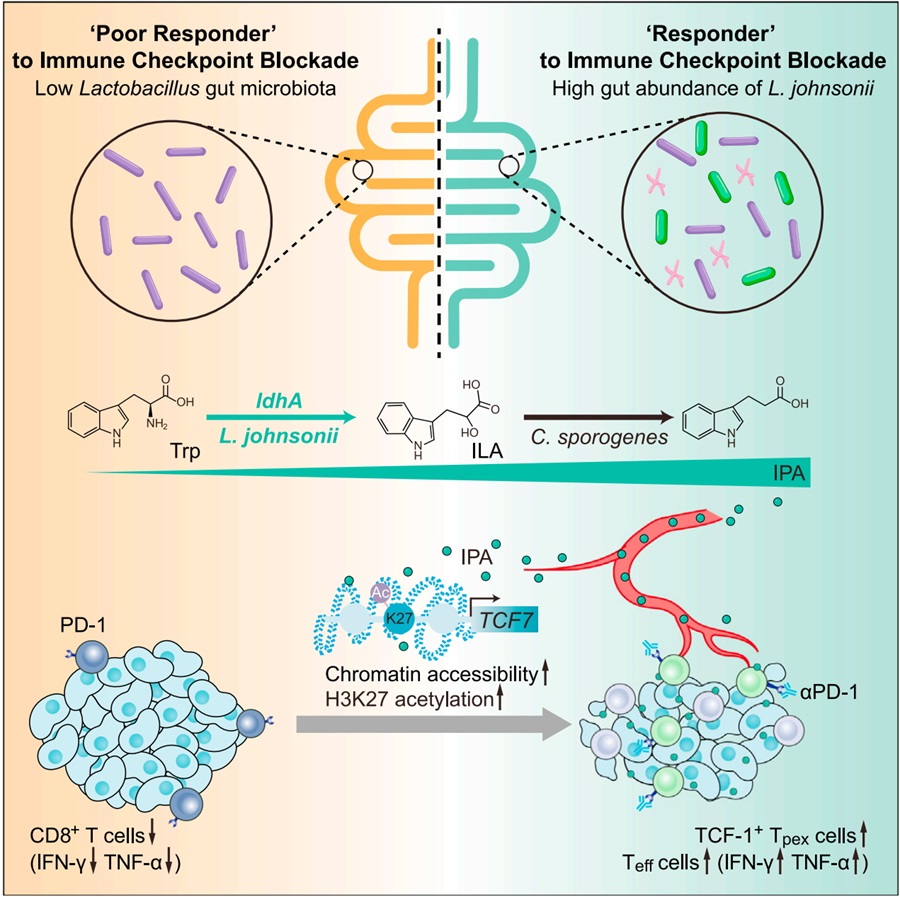
In summary, this work reveals a tryptophan derivative cooperatively produced by different bacteria, elucidates the mechanism by which IPA regulates key transcription factors of immune stemness through histone acetylation modifications, and validates the sensitizing immunotherapeutic effects of IPA at the pan-cancer level. This bacterial-derived metabolite-host immunomodulatory pathway provides a potential new idea for sensitizing immunotherapy.
Professor Liangjing Wang and professor Shujie Chen are the corresponding authors of this paper. Dingjiacheng Jia, Qiwen Wang, Yadong Qi, Yao Jiang, and Jiamin He, postgraduate students of Zhejiang University, were the co-first authors. This study was supported and assisted by Professor Jianmin Si, Professor Di Wang, Professor Yongqun Zhu, and Professor Lie Wang of Zhejiang University. This project was financially supported by the National Foundation of Natural Science of China, the Key program of Natural Science Foundation of Zhejiang Province and the National Key Research and Development Program of China.
Links: https://doi.org/10.1016/j.cell.2024.02.022
Research highlighted by Cancer Discovery: https://doi.org/10.1158/2159-8290.CD-RW2024-047
On March 2024, Professor Liangjing Wang's team (Second Affiliated Hospital of Zhejiang University School of Medicine) and Professor Shujie Chen's team (Sir Run Run Shaw Hospital, Zhejiang University) jointly published a research article in Cell entitled ‘Microbial metabolite enhances immunotherapy efficacy by modulating T-cell stemness in pan-cancer’. The researchers report that the abundance of commensal Lactobacillus johnsonii is positively correlated with the responsiveness of ICB. Supplementation with Lactobacillus johnsonii or tryptophan-derived metabolite indole-3-propionic acid (IPA) enhances the efficacy of CD8+ T cells-mediated αPD-1 immunotherapy. Mechanistically, Lactobacillus johnsonii collaborates with Clostridium sporogenes to produce IPA. IPA modulates the stemness program of CD8+ T cells and facilitates the generation of Tpex cells by increasing H3K27 acetylation at the super-enhancer region of Tcf7. IPA improves ICB responsiveness at the pan-cancer level including melanoma, breast cancer and colorectal cancer. Collectively, the findings identify a microbial metabolite-immune regulatory pathway and suggest a potential microbial-based adjuvant approach to improve the responsiveness of immunotherapy.
In this study, the authors firstly found that there were ‘responsive’ and ‘poorly responsive’ mice to immunotherapy similar to clinical practice, and verified that gut microbiota can influence the efficacy of tumor immunotherapy based on FMT; through sequencing and screening of gut microbiota, the researchers independently isolated a strain of Lactobacillus johnsonii (L. j), which increased the efficacy of CD8+ T-cell infiltration and sensitization of anti-PD1 therapy; the researchers then explored the efficacy components of L. j, compared with heat-inactivated and ultrasonically fragmented bacteria, the culture supernatant of L. j had similar effects as live L. j, suggesting that bacterial metabolites may play a key role; by using LC-MS/MS, the researchers targeted the tryptophan metabolic pathway and the key ingredient: indole-3-propionic acid (IPA).
The researchers used Rag1-/- mice, anti-CD8 neutralizing antibody, and adoptive cell transfer therapy to validate the sensitizing immunotherapeutic effects of L. j and its derived IPA dependent on CD8+ T cells, and further sorted CD8+ T cells infiltrated in tumors of mice for single-cell RNA sequencing (scRNA-seq), single-cell TCR sequencing (scTCR-seq) and single-cell Assay for Targeting Accessible-Chromatin with high-throughout sequencing (scATAC-seq); gavage IPA significantly increased the proportion of ‘stem cell-like’ Tpex cells and effector CD8+ T cells, Tcf7-/- mice verified that IPA requires Tpex cells for sensitizing immunotherapeutic effects; combined with single-cell transcriptomic pathway enrichment and scATAC-seq, the authors found that IPA regulates chromatin opening in Tpex cells through histone acetylation modifications; western blot, ChIP, CUT & RUN, and CUT & Tag sequencing further revealed that IPA increases H3K27 acetylation in the Tcf7 super-enhancer region.
The authors then explored how L. j produces IPA; interestingly, IPA was not detected in L. j culture supernatants in vitro, but instead the upstream metabolite of IPA, indole-3-lactic acid (ILA) was detected; based on the antibiotic cocktail model and intraperitoneal injection model, the authors hypothesized that ILA produced by L. j is further metabolized to IPA by other gut microbiota in vivo and verified this in germ-free mice model; the authors also predicted that ldhA, was the key enzyme for ILA production by L. j and transformed a ldhA expressional vector into E. coli. E. coli overexpressing ldhA increased IPA production and sensitized immunotherapeutic effects. Finally, the investigators verified the regulatory effects of IPA on Tpex cells and immunotherapy in a variety of mouse tumor models (both breast cancer and melanoma transplantable tumor model, mammary fat pad orthotopic implantation model, MMTV-PyMT spontaneous breast cancer model and cecum orthotopic implantation model) and in organoids derived from colorectal cancer patients.

In summary, this work reveals a tryptophan derivative cooperatively produced by different bacteria, elucidates the mechanism by which IPA regulates key transcription factors of immune stemness through histone acetylation modifications, and validates the sensitizing immunotherapeutic effects of IPA at the pan-cancer level. This bacterial-derived metabolite-host immunomodulatory pathway provides a potential new idea for sensitizing immunotherapy.
Professor Liangjing Wang and professor Shujie Chen are the corresponding authors of this paper. Dingjiacheng Jia, Qiwen Wang, Yadong Qi, Yao Jiang, and Jiamin He, postgraduate students of Zhejiang University, were the co-first authors. This study was supported and assisted by Professor Jianmin Si, Professor Di Wang, Professor Yongqun Zhu, and Professor Lie Wang of Zhejiang University. This project was financially supported by the National Foundation of Natural Science of China, the Key program of Natural Science Foundation of Zhejiang Province and the National Key Research and Development Program of China.
Links: https://doi.org/10.1016/j.cell.2024.02.022
Research highlighted by Cancer Discovery: https://doi.org/10.1158/2159-8290.CD-RW2024-047


