Researchers from Sun Yat-sen University and Guangzhou Laboratory reveal a new mechanism of immune evasion of SARS-CoV-2 Omicron
Source:Hui Zhang
2024-03-26
As of January 2024, COVID-19, caused by SARS-CoV-2 infection, has resulted in over 774 million confirmed cases and over 7.01 million deaths. SARS-CoV-2 exhibits rapid mutation rates, enabling the appearance of new variant strains replacing older ones globally in a very short period. Currently, Omicron subvariants HV.1 and JN.1 are rapidly replacing early Omicron subtypes BA.2, BA.2.75, and XBB.1. Clinical symptoms of infections with Omicron are milder compared to previous variants, such as Delta. However, the weak immunogenicity of Omicron-specific vaccines severely hampens the development of effective vaccines.
On March 7, 2024, Sun Yat-sen University researchers Drs. Hui Zhang, Xin He, Yiwen Zhang, and Ran Chen, in collaboration with Guangzhou Laboratory Researchers Drs. Jincun Zhao and Xuepeng Wei, published a research article titled "The receptor binding domain of SARS-CoV-2 Omicron subvariants targets Siglec-9 to decrease its immunogenicity by preventing macrophage phagocytosis" in Nature Immunology.

Using reverse mutation combined with nanoparticle assembly technology, the research group systematically screened the amino acid mutations in the Omicron Spike protein RBD region for their impact on immunogenicity. The results showed that the substitution of phenylalanine (F) with serine (S) at position 375 of the Omicron RBD, i.e., F375S, significantly enhanced the immunogenicity of RBD. Furthermore, it was found that the FAPFFAF sequence at positions 371-377 of the Omicron RBD strongly inhibited the phagocytic function of macrophages towards RBD nanoparticles or Spike pseudovirus particles containing this sequence. The study suggests that the Omicron RBD inhibits phagocytic function and antigen presentation by enhancing binding to the immune checkpoint Siglec-9 on macrophages, leading to immune evasion, thereby providing novel theoretical support for vaccine design to overcome immune escape.
To further enhance vaccine immunogenicity and induce broad-spectrum immune responses, researchers developed BA.5 (F375S/V486F/Q493R) based on RBD structure and site conservation analysis, named BA.5(SR). BA.5(SR)/Delta bivalent nanoparticle vaccine, composed of BA.5(SR) and Delta RBD, induced potent and broad-spectrum neutralizing antibodies in experiments on mice, New Zealand rabbits, and rhesus monkeys, indicating significant potential in addressing threats from Omicron and other variant strains.
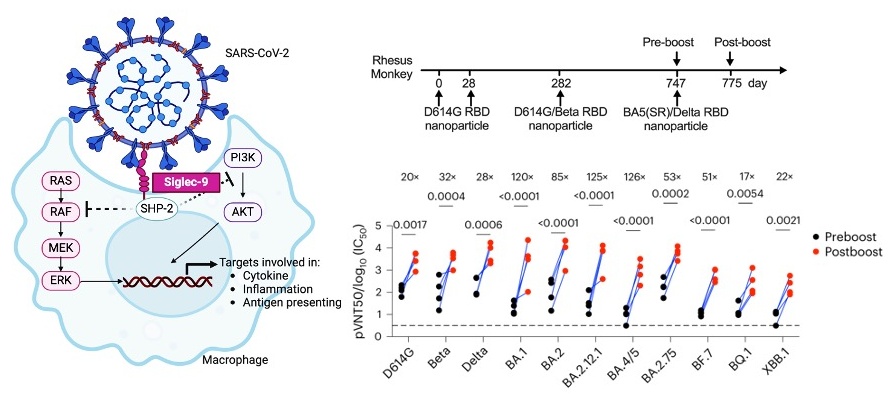
By revealing a novel function of Omicron directly inhibiting macrophage phagocytosis and antigen presentation as a new mechanism of immune evasion, the newly designed COVID-19 vaccine demonstrates potent and broad-spectrum protective activity, providing new avenues for vaccine development. The findings of this study are expected to play a key role in future vaccine strategies, providing more reliable protection for global public health security.
Dr. Xin He, and graduate students including Xiantao Zhang, Bolin Wu, Jieyi Deng, Yongli Zhang from Zhongshan School of Medicine at Sun Yat-sen University, and Dr. Airu Zhu from Guangzhou Medical University are co-first authors. Drs. Hui Zhang, Yiwen Zhang, Ran Chen, Xuepeng Wei, and Jincun Zhao served as co-corresponding authors. Dr. Jun Chen from Sun Yat-sen University constructed the Siglec-9/E knockout mouse model. This research was funded by the Key Research and Development Program of the Ministry of Science and Technology, the Major Projects and General Programs of the National Natural Science Foundation of China, as well as key research and development projects of Guangdong Province.
Links: https://www.nature.com/articles/s41590-024-01776-2
On March 7, 2024, Sun Yat-sen University researchers Drs. Hui Zhang, Xin He, Yiwen Zhang, and Ran Chen, in collaboration with Guangzhou Laboratory Researchers Drs. Jincun Zhao and Xuepeng Wei, published a research article titled "The receptor binding domain of SARS-CoV-2 Omicron subvariants targets Siglec-9 to decrease its immunogenicity by preventing macrophage phagocytosis" in Nature Immunology.

Using reverse mutation combined with nanoparticle assembly technology, the research group systematically screened the amino acid mutations in the Omicron Spike protein RBD region for their impact on immunogenicity. The results showed that the substitution of phenylalanine (F) with serine (S) at position 375 of the Omicron RBD, i.e., F375S, significantly enhanced the immunogenicity of RBD. Furthermore, it was found that the FAPFFAF sequence at positions 371-377 of the Omicron RBD strongly inhibited the phagocytic function of macrophages towards RBD nanoparticles or Spike pseudovirus particles containing this sequence. The study suggests that the Omicron RBD inhibits phagocytic function and antigen presentation by enhancing binding to the immune checkpoint Siglec-9 on macrophages, leading to immune evasion, thereby providing novel theoretical support for vaccine design to overcome immune escape.
To further enhance vaccine immunogenicity and induce broad-spectrum immune responses, researchers developed BA.5 (F375S/V486F/Q493R) based on RBD structure and site conservation analysis, named BA.5(SR). BA.5(SR)/Delta bivalent nanoparticle vaccine, composed of BA.5(SR) and Delta RBD, induced potent and broad-spectrum neutralizing antibodies in experiments on mice, New Zealand rabbits, and rhesus monkeys, indicating significant potential in addressing threats from Omicron and other variant strains.

By revealing a novel function of Omicron directly inhibiting macrophage phagocytosis and antigen presentation as a new mechanism of immune evasion, the newly designed COVID-19 vaccine demonstrates potent and broad-spectrum protective activity, providing new avenues for vaccine development. The findings of this study are expected to play a key role in future vaccine strategies, providing more reliable protection for global public health security.
Dr. Xin He, and graduate students including Xiantao Zhang, Bolin Wu, Jieyi Deng, Yongli Zhang from Zhongshan School of Medicine at Sun Yat-sen University, and Dr. Airu Zhu from Guangzhou Medical University are co-first authors. Drs. Hui Zhang, Yiwen Zhang, Ran Chen, Xuepeng Wei, and Jincun Zhao served as co-corresponding authors. Dr. Jun Chen from Sun Yat-sen University constructed the Siglec-9/E knockout mouse model. This research was funded by the Key Research and Development Program of the Ministry of Science and Technology, the Major Projects and General Programs of the National Natural Science Foundation of China, as well as key research and development projects of Guangdong Province.
Links: https://www.nature.com/articles/s41590-024-01776-2


