Qiang Zou’s group reveals how lactate facilitates CTLA-4 RNA splicing and expression in tumor-infiltrating Treg cells to maintain its immunosuppressive function
Source:Qiang Zou
2024-03-25
On February 27, 2024, Qiang Zou's team at Shanghai Institute of Immunology, Shanghai Jiao Tong University School of Medicine (SJTUSM), published a research paper in Immunity, entitled “Lactate modulates RNA splicing to promote CTLA-4 expression in tumor-infiltrating regulatory T cells”. This research reveals that lactate modulates the immunosuppressive functions of tumor-infiltrating Treg cells through the lactate-Foxp3-USP39-CTLA-4 pathway.
Although CTLA-4 blockade antibody mediates intratumoral Treg cell depletion to induce durable antitumor immune responses and tumor regression, its efficacy varies among patients. Lactate accumulation, a hallmark of the metabolically altered tumor microenvironment, plays a critical role in sustaining the function of tumor-infiltrating Treg cells. It has been demonstrated that lactate is involved in the regulation of the PD-1 monotherapy efficacy, whether it modulates CTLA-4 expression in Treg cells to impact the CTLA-4 blockade therapy responsessiveness is worth exploring.
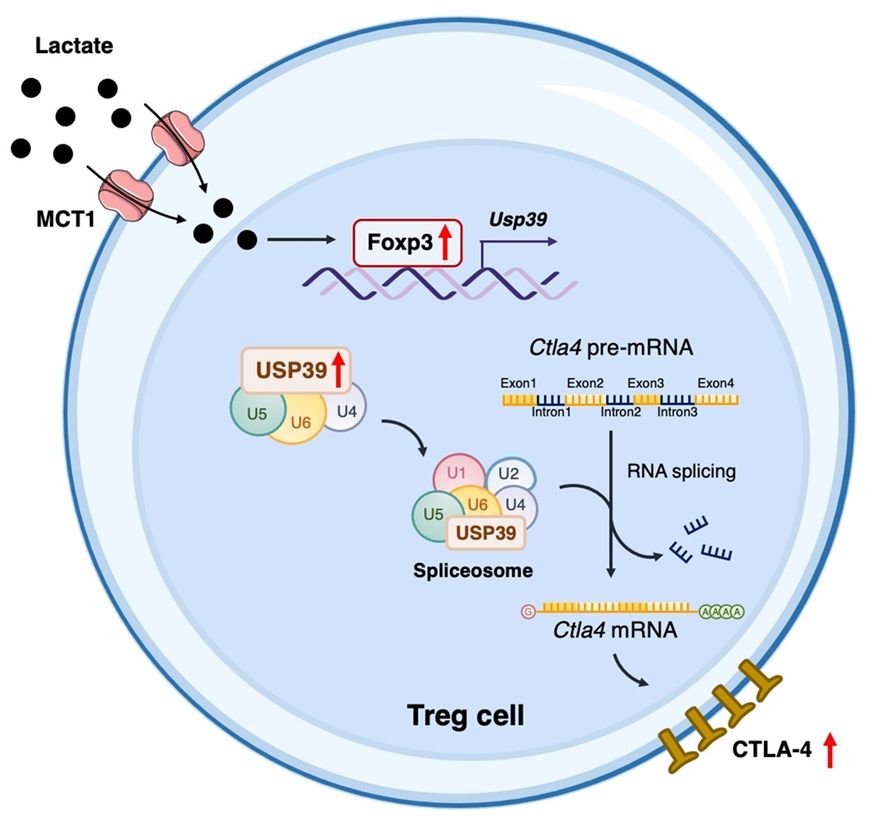
Utilizing murine subcutaneous transplantation tumor models, the research team found that uptake of lactate by intratumoral Treg cells contributed to the efficacy of CTLA-4 blockade therapy. Through further analyzing published scRNA-seq databases and verifying with tumor-infiltrating Treg cells from colorectal cancer (CRC) patients, they identified a positive correlation between the expression of lactate transporter SLC16A1 and the expression of USP39, which is a component of the RNA splicing machinery. This correlation, along with the association between USP39 expression and Treg cell signatures in tumor-infiltrating Treg cells from CRC patients, underscores the importance of USP39. Indeed, tumor-bearing Treg cell conditional knockout of Usp39 mice displayed an improved anti-tumor immune response.
Further investigations revealed that lactate uptake by Treg cells boosts the expression of Treg cell lineage-specific transcription factor Foxp3, thus increasing USP39 expression. USP39 facilitated the pre-mRNA maturation and the expression of CTLA-4, thereby reinforcing Treg cell immunosuppressive function. In addition, lactate uptake-mediated upregulation of CTLA-4 in Treg cells improved the elimination efficiency of CTLA-4 antibody-mediated tumor-infiltrating Treg cell clearance, thus augmenting the therapeutic efficacy of CTLA-4 monotherapy.
In summary, this study uncovers the intricate relationship between lactate and RNA splicing in Treg cells, positioning USP39 as a key mediator of Treg cell adaptation to their environment. These findings suggest that lactate accumulation in the tumor microenvironment could positively impact the efficacy of CTLA-4 monotherapy, offering new avenues for enhancing antitumor immunity.
Prof. Qiang Zou and Youqiong Ye from Shanghai Institute of Immunology, Prof. Ren Zhao at Ruijin hospital, and Prof. Zhi-yu Ni at Hebei University of Engineering are the co-corresponding authors of this paper. PhD student Rui Ding, Associate research fellow Xiaoyan Yu at Shanghai Institute of Immunology, and Prof. Zhilin Hu at Nanjing Medical University are the co-first authors of this paper. This work was supported by the Program of the National Natural Science Foundation of China and the National Key Research and Development Program of China.
Links: https://linkinghub.elsevier.com/retrieve/pii/S1074-7613(24)00045-1
Although CTLA-4 blockade antibody mediates intratumoral Treg cell depletion to induce durable antitumor immune responses and tumor regression, its efficacy varies among patients. Lactate accumulation, a hallmark of the metabolically altered tumor microenvironment, plays a critical role in sustaining the function of tumor-infiltrating Treg cells. It has been demonstrated that lactate is involved in the regulation of the PD-1 monotherapy efficacy, whether it modulates CTLA-4 expression in Treg cells to impact the CTLA-4 blockade therapy responsessiveness is worth exploring.
Utilizing murine subcutaneous transplantation tumor models, the research team found that uptake of lactate by intratumoral Treg cells contributed to the efficacy of CTLA-4 blockade therapy. Through further analyzing published scRNA-seq databases and verifying with tumor-infiltrating Treg cells from colorectal cancer (CRC) patients, they identified a positive correlation between the expression of lactate transporter SLC16A1 and the expression of USP39, which is a component of the RNA splicing machinery. This correlation, along with the association between USP39 expression and Treg cell signatures in tumor-infiltrating Treg cells from CRC patients, underscores the importance of USP39. Indeed, tumor-bearing Treg cell conditional knockout of Usp39 mice displayed an improved anti-tumor immune response.
Further investigations revealed that lactate uptake by Treg cells boosts the expression of Treg cell lineage-specific transcription factor Foxp3, thus increasing USP39 expression. USP39 facilitated the pre-mRNA maturation and the expression of CTLA-4, thereby reinforcing Treg cell immunosuppressive function. In addition, lactate uptake-mediated upregulation of CTLA-4 in Treg cells improved the elimination efficiency of CTLA-4 antibody-mediated tumor-infiltrating Treg cell clearance, thus augmenting the therapeutic efficacy of CTLA-4 monotherapy.
In summary, this study uncovers the intricate relationship between lactate and RNA splicing in Treg cells, positioning USP39 as a key mediator of Treg cell adaptation to their environment. These findings suggest that lactate accumulation in the tumor microenvironment could positively impact the efficacy of CTLA-4 monotherapy, offering new avenues for enhancing antitumor immunity.

Prof. Qiang Zou and Youqiong Ye from Shanghai Institute of Immunology, Prof. Ren Zhao at Ruijin hospital, and Prof. Zhi-yu Ni at Hebei University of Engineering are the co-corresponding authors of this paper. PhD student Rui Ding, Associate research fellow Xiaoyan Yu at Shanghai Institute of Immunology, and Prof. Zhilin Hu at Nanjing Medical University are the co-first authors of this paper. This work was supported by the Program of the National Natural Science Foundation of China and the National Key Research and Development Program of China.
Links: https://linkinghub.elsevier.com/retrieve/pii/S1074-7613(24)00045-1


