Dr. Jie Zhou and Dr. Heping Xu’s groups identify Dopamine as negative regulator of type 2 innate lymphoid cells in allergic asthma
Source:Jie Zhou
2023-02-20
Allergic asthma is one of the most common chronic inflammatory diseases which is characterized with hyper-responsiveness and airway remodeling due to repeated allergens exposure. Currently, the overall prevalence of asthma in Chinese adults is 4.2%, representing 45.7 million people. Accumulating evidence has demonstrated a critical role of group 2 innate lymphoid cells (ILC2s) in both the initiation and progression of allergic asthma. ILC2s express a variety of receptors of soluble factors, such as cytokines, chemokines, nutrients and hormones, to receive environmental cues and participate in tissue homeostasis and inflammation by secretion of type 2 cytokines. Interestingly, emerging evidence indicates that neuronal signals act as a regulator of group 2 innate lymphoid cells (ILC2s), but the underlying mechanisms remains largely unclear.
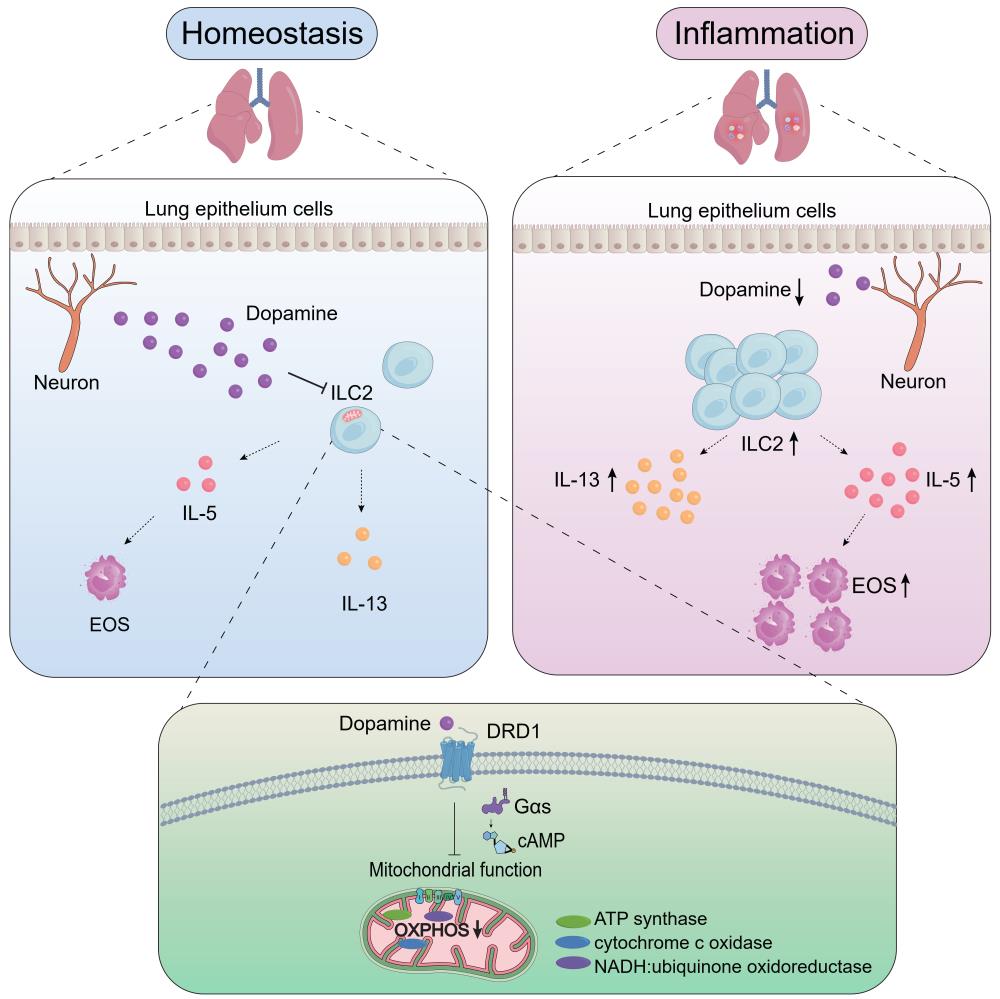
On Feb. 14th, 2023, Dr. Jie Zhou’s group from Tianjin Medical University and Dr. Heping Xu’s group from Westlake University published a research article entitled ” Dopamine inhibits type 2 innate lymphoid cell-driven allergic lung inflammation by dampening mitochondrial activity” on Immunity. They identified neurotransmitter dopamine as a negative regulator of ILC2s. In this study, they found that in both asthmatic patients and patients with Parkinson’s disease (PD), the concentration of plasma dopamine was negatively correlated with the numbers of circulating ILC2 and compromised pulmonary function. These clinical observations indicate a potential link between dopamine and ILC2 responses. Utilization of both in vitro cell culture and in vivo animal models, they demonstrated that dopamine efficiently restricted ILC2 activation, including cell proliferation and effector cytokine production in response to alarmin rIL-33. Intranasal administration of rIL-33 reduced dopamine levels in lungs and dopaminergic neurons innervating the lung tissue. ILC2 abundantly expressed dopamine receptor DRD1. Pharmacological inhibition or conditional genetic ablation of DRD1 enhanced ILC2 responses. Using mixed bone marrow chimera and adoptive transfer of ILC2s into immunodeficient mice, they showed that the regulation of ILC2 by dopamine was cell intrinsic. As consequences of the diminished ILC2 responses, intranasal administration of dopamine caused clear remission of allergic airway inflammation. Combination of single-cell/ bulk RNA sequencing and seahorse assays, the authors showed that impaired mitochondrial oxidative phosphorylation (OXPHOS) represented the underlying mechanism of dopamine in ILC2s. These findings reveal a previously unrecognized role of dopamine in the restriction of innate type 2 immunity in lungs.
In the same issue of Immunity, this paper has been highlighted in a Previews entitled “Breathing easy, Dopamine quenches the ILC2 flames”. The experts commented “uncover an essential role for dopamine in inhibiting ILC2 function via metabolic restriction, thereby ameliorating key features of asthma pathogenesis”, “demonstrate that dopamine represents an inhibitory regulator of ILC2 responses in acute allergic airway inflammation”.
This study was supported by National Natural Science Foundation of China.
Drs. Yingjiao Cao and Yu Li are the equally contributing first authors. Drs. Jie Zhou (Tianjin Institute of Immunology/Tianjin Medical University) and Heping Xu (Westlake University) are the co-corresponding authors.
Article: https://doi.org/10.1016/j.immuni.2022.12.017
Previews: https://doi.org/10.1016/j.immuni.2023.01.019
On Feb. 14th, 2023, Dr. Jie Zhou’s group from Tianjin Medical University and Dr. Heping Xu’s group from Westlake University published a research article entitled ” Dopamine inhibits type 2 innate lymphoid cell-driven allergic lung inflammation by dampening mitochondrial activity” on Immunity. They identified neurotransmitter dopamine as a negative regulator of ILC2s. In this study, they found that in both asthmatic patients and patients with Parkinson’s disease (PD), the concentration of plasma dopamine was negatively correlated with the numbers of circulating ILC2 and compromised pulmonary function. These clinical observations indicate a potential link between dopamine and ILC2 responses. Utilization of both in vitro cell culture and in vivo animal models, they demonstrated that dopamine efficiently restricted ILC2 activation, including cell proliferation and effector cytokine production in response to alarmin rIL-33. Intranasal administration of rIL-33 reduced dopamine levels in lungs and dopaminergic neurons innervating the lung tissue. ILC2 abundantly expressed dopamine receptor DRD1. Pharmacological inhibition or conditional genetic ablation of DRD1 enhanced ILC2 responses. Using mixed bone marrow chimera and adoptive transfer of ILC2s into immunodeficient mice, they showed that the regulation of ILC2 by dopamine was cell intrinsic. As consequences of the diminished ILC2 responses, intranasal administration of dopamine caused clear remission of allergic airway inflammation. Combination of single-cell/ bulk RNA sequencing and seahorse assays, the authors showed that impaired mitochondrial oxidative phosphorylation (OXPHOS) represented the underlying mechanism of dopamine in ILC2s. These findings reveal a previously unrecognized role of dopamine in the restriction of innate type 2 immunity in lungs.

Cao et al. Immunity, 2023
In the same issue of Immunity, this paper has been highlighted in a Previews entitled “Breathing easy, Dopamine quenches the ILC2 flames”. The experts commented “uncover an essential role for dopamine in inhibiting ILC2 function via metabolic restriction, thereby ameliorating key features of asthma pathogenesis”, “demonstrate that dopamine represents an inhibitory regulator of ILC2 responses in acute allergic airway inflammation”.
This study was supported by National Natural Science Foundation of China.
Drs. Yingjiao Cao and Yu Li are the equally contributing first authors. Drs. Jie Zhou (Tianjin Institute of Immunology/Tianjin Medical University) and Heping Xu (Westlake University) are the co-corresponding authors.
Article: https://doi.org/10.1016/j.immuni.2022.12.017
Previews: https://doi.org/10.1016/j.immuni.2023.01.019


